Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Fig. 2
The proportion of the patients who achieved well-controlled asthma in week 12. The proportion test (one-sided) for proving non-inferiority of MON-400BUD vs 800BUD in per-protocol population without the last observation carried forward (LOCF) approach and intention-to-treat set with LOCF, with a predetermined non-inferiority margin of -17.2% for the difference in the rates of well-controlled asthma between treatments. MON-400BUD, montelukast added to low-dose inhaled budesonide; 800BUD, medium-dose inhaled budesonide; PP, per-protocol; ITT, intention-to-treat; LOCF, last observation carried forward; CI, confidence interval.
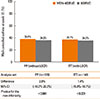
Fig. 3
The proportions of asthma control status in the MON-400BUD (A) and 800BUD (B) groups during the study period. MON-400BUD, montelukast added to low-dose inhaled budesonide; 800BUD, medium-dose inhaled budesonide.

Fig. 4
(A) Asthma Control Test (ACT) scores in the 2 groups. The error bars indicate standard deviation of the means. (B) The proportion of patients with ACT <20 at each time point.

Table 1
Clinical characteristics of the study subjects
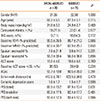
The t test was used for analysis of continuous variables and Fisher's exact test was used for categorical variables.
MON-400BUD, combination therapy consisting of montelukast and low-dose inhaled budesonide; 800BUD, monotherapy consisting of a medium dose of inhaled budesonide; INS, intranasal corticosteroid; FEV1%, percentage of expected volume exhaled at the end of the first second of forced expiration; ACT, asthma control test; AQoL, asthma-specific quality of life questionnaire; PFS, physical functioning scale.
Table 2
Changes in pulmonary function, sputum differential count, and physical functioning scales

MON-400BUD, combination therapy consisting of montelukast and low-dose inhaled budesonide; 800BUD, monotherapy consisting of a medium dose of inhaled budesonide; FEV1%, percentage of expected volume exhaled at the end of the first second of forced expiration; MMEF%, maximal expiratory flow rate; PFS, physical functioning scale.
Table 3
Determinants of asthma control status over 12 weeks of treatment

*ANOVA and Pearson's χ2; †Multivariate ordinal regression analysis with reference to the uncontrolled group.
MON-400BUD, combination therapy consisting of montelukast and low-dose inhaled budesonide; 800BUD, monotherapy consisting of a medium dose of inhaled budesonide; BMI, body mass index; FEV1%, percentage of expected volume exhaled at the end of the first second of forced expiration; ACT, asthma control test; AQoL, asthma-specific quality of life questionnaire; PFS, physical functioning scale; GDS, geriatric depression score.
Table 4
Total experiences of local adverse events and asthma exacerbation during 12 weeks of treatment

Fisher's exact test was used to compare numbers of events between two groups.
MON-400BUD, combination therapy consisting of montelukast and low-dose inhaled budesonide; 800BUD, monotherapy consisting of a medium dose of inhaled budesonide; #, number; OPD, outpatient department; ED, emergency department.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download