Abstract
Purpose
Rhinitis and conjunctivitis are common diseases worldwide that are frequently associated. Nevertheless, the risk factors for rhinoconjunctivitis are not well-described and the impact of conjunctivitis on rhinitis and asthma in children remains unknown. This study explored the different risk factors and evaluated the burden of rhinoconjunctivitis among adolescents.
Methods
This was a cross-sectional study conducted on a random sample of schoolchildren, aged 10-17 years, using skin prick tests and a self-administered questionnaire on respiratory health investigating the impact of rhinitis and rhinoconjunctivitis on daily activities.
Results
A complete evaluation was obtained for 2,150 children. The prevalence of rhinitis alone was 18.2% and rhinitis associated with conjunctivitis was 20.5%. Rhinoconjunctivitis was more frequently associated with females, a parental history of atopy, domestic exposure to mold/dampness, passive smoke exposure, and reported truck traffic in residential streets. Moreover, rhinoconjunctivitis was associated with a higher level of allergic sensitization. The prevalence of current asthma was 1.7% in subjects without rhinitis or rhinoconjunctivitis, 5.1% in rhinitis and 10.7% in rhinoconjunctivitis. In a logistic model, rhinoconjunctivitis yielded a 2-fold risk for current asthma with respect to rhinitis. Subjects with rhinoconjunctivitis had poorer quality of life (QoL); there was an impact on daily activities in 4.6% of rhinitis and 10.7% of rhinoconjunctivitis.
Rhinitis and rhinoconjunctivitis are common diseases worldwide in both adults and children, and their prevalence appears to be increasing.1 There are wide variations in the prevalence of rhinitis and rhinoconjunctivitis in different countries and in different regions within the same country.2 Furthermore, ocular symptoms are common and contribute to the burden of rhinitis.3
Ocular allergic symptoms are strongly associated with allergic rhinitis, even though they are often underdiagnosed-at least in mild forms-and consequently undertreated. Conjunctivitis is now increasingly recognized as a distinct disorder that imposes its own burden on both medical costs and a patient's quality of life (QoL).4
Rhinitis and conjunctivitis can be associated with other inflammatory diseases, such as asthma.4 The link between the upper and the lower respiratory tracts (united airway disease) has been established previously5; in this context, conjunctivitis should be considered part of this entity because the allergic response involves the conjunctival surface as well as the respiratory tract.2 Consequently, the Allergic Rhinitis and its Impact on Asthma (ARIA) initiative has now recognized conjunctivitis as a frequent comorbidity of allergic rhinitis.6 Moreover, although rhinitis is a well-recognized risk factor for asthma and is associated with increased asthma severity,7 little is known on the role of conjunctivitis as an additional risk factor.
Few studies have explored the impact of allergic conjunctivitis on asthma.3 Therefore, using a population sample evaluated during a cross-sectional study performed on schoolchildren, we investigated (1) whether rhinitis and rhinoconjunctivitis are associated with different risk factors, (2) the impact of rhinitis and rhinoconjunctivitis on asthma, and (3) the burden of rhinoconjunctivitis among adolescents.
This is a cross-sectional study conducted on a random sample of schoolchildren, aged 10-17 years, living in the city of Palermo in the Mediterranean area of Southern Italy, between November 2005 and May 2006.8 Students completed a questionnaire based on SIDRIA and ISAAC surveys9,10 and underwent skin prick tests (SPTs) at school. A total of 2,150 children were evaluated. The study was approved by the Institutional Ethics Committee. All parents of invited adolescents signed a written informed consent. According to Italian law, individual privacy was respected.
The self-administered questionnaire was completed by adolescents at school regarding respiratory allergic symptoms, diseases and personal information.9 The core questionnaire modules of ISAAC for 13-14 year olds for wheezing and rhinitis were used.11 According to ISAAC methods,11 a child's history of asthma was defined as a positive answer to the question "Have you ever had asthma?"; Rhinitis was defined as a positive answer to the question "Have you ever had a problem with sneezing, or runny, or blocked nose apart from common cold or flu in the last 12 months?"; Conjunctivitis was defined by the question "In the past 12 months, has this nose problem been accompanied by itching and/or watering eyes?". Based on these criteria, we identified the following subgroups: subjects with asthma, subjects with rhinitis, subjects with rhinoconjunctivitis, and asymptomatic children without asthma or rhinoconjunctivitis. Current asthma was defined as asthma plus at least one wheezing episode in the last 12 months. Moreover, subjects with current asthma were asked if at least one visit to the hospital emergency department because of an asthma exacerbation had occurred.
Rhinitis-related impact on QoL was considered if the answer to the question "In the last 12 months, how much did nasal symptoms interfere with your daily activities?" was "moderately/highly".
Information on possible confounders or effect modifiers was also collected. Domestic mold/dampness exposure was evaluated based on the question: "Have you ever seen mold/dampness/fungi on the walls or on the ceiling of your bedroom?" Environmental tobacco smoke (ETS) exposure was assessed based on the question "Are there smokers at home?" Self reported traffic exposure was recorded as the frequency of trucks passing on a residential street on weekdays (never/rare/frequent/constant) and subjects were considered exposed if they answered "frequent" or "constant."
Parental history for atopy was defined as at least one parent with a personal history of allergic respiratory diseases (asthma and/or rhinitis). Household crowding index (HCI) was defined as the total number of co-residents per household, excluding newborn infants, divided by the total number of rooms, excluding the kitchen and bathrooms. HCI was dichotomized at the level of its 50th percentile (< 1.0 and ≥ 1.0) and used as an index of socioeconomic status.12
Height and weight were measured in all children in standing position without shoes using a stadiometer and an electronic digital scale: body mass index (BMI) was computed as weight/height2 (kg/m2). Overweight-obese children were defined following the gender- and age-specific BMI cut-offs by Cole.13
SPTs were performed following EAACI recommendations14 with a panel including 8 common aeroallergens, as well as positive and negative controls (Stallergènes Italia S.r.l., Milan, Italy). Allergic sensitization was defined as the presence of at least one positive skin prick test. Atopic index (AI) was computed as the number of the individual positive SPT and classified as follows: 0, no allergic sensitization; 1, 1 positive SPT; 2, 2 positive SPTs; and 3, 3 or more positive SPTs. Allergens were grouped in perennial (Parietaria judaica, Dermatophagoides, dog and cat dander, Blattella germanica, and alternaria) and seasonal (grass mix and olive).
Frequency distribution of variables was evaluated using the χ2 test. To identify independent variables that influence the risk for a dependent variable, adjusting for confounders, logistic regression models were used and odds ratios (OR) with corresponding 95% confidence intervals (CI) were calculated for all predictors.
Computations were performed using the StatView statistical software package (SAS Institute, Cary, NC, USA). A probability level <0.05 was considered to indicate significance.
Sample characteristics (2,150 subjects: M 49.2%) are shown in Table 1. Excluding asthma, no chronic pulmonary disease was reported; none of the children were active smokers. The prevalence of allergic sensitization was 39.2% (45.0% in males and 33.5% in females, P < 0.0001, χ2 test).
In the overall sample, we found a prevalence of 18.2% (391/2,150) for rhinitis (17.3% alone and 0.9% in association with CA) and of 20.5% (440/2,150) for rhinoconjunctivitis (18.3% alone and 2.2% in association with CA) (Table 2). The prevalence rate of CA was 4.2% (90/2,150). Because CA was strongly associated with rhinitis (74.4% of subjects with CA had rhinitis or rhinoconjunctivitis), to evaluate factors influencing the prevalence of rhinitis and rhinoconjunctivitis we excluded subjects with CA from the analyses. On the remaining 2,060 subjects, the proportion of rhinitis was 37.1%; 18.0% isolated and 19.1% in association with conjunctivitis.
There were fewer females than males among subjects without rhinitis or rhinoconjunctivitis (49.5%) and those with rhinitis (48.2%), while there were more females than males with rhinoconjunctivitis (59.3%, P=0.0014, χ2 test). Similarly, parental history for atopy, domestic exposure to mold/dampness, ETS, and reported truck traffic in the residential streets were more frequent in rhinoconjunctivitis (Table 3). Rhinoconjunctivitis was significantly more frequent in those with higher AI than rhinitis (Table 4). In a multiple logistic regression model, high truck traffic, mold/dampness, and ETS were independent risk factors for the association of conjunctivitis with rhinitis (Table 5). In the same model, sensitization to perennial allergens and to perennial plus seasonal allergens increased the risk of having conjunctivitis, while isolated sensitization to seasonal allergens did not increase the risk for conjunctivitis.
In the overall samples (N=2,150), the prevalence of current asthma was 1.7% among subjects without rhinitis or rhinoconjunctivitis, 5.1% among those with rhinitis, and 10.7% among those with rhinoconjunctivitis (P<0.0001, χ2 test). Among subjects with current asthma, 15.6% reported a hospitalization due to asthma exacerbation in the last 12 months. In a multiple logistic regression model, rhinoconjunctivitis produced a twofold independent risk for current asthma with respect to rhinitis alone, which was larger than the risk produced by allergic sensitization, parental history for asthma, and heavy traffic (Table 6). Consequently, combinations of allergic sensitization, rhinitis, and rhinoconjunctivitis strongly increased the frequency of current asthma, as shown in Table 7. Hospitalization for asthma exacerbation was reported in 0.5% of children without rhinitis or rhinoconjunctivitis, 1.3% in rhinitis, and 2.0% in rhinoconjunctivitis (P=0.008, χ2 test).
Subjects with rhinoconjunctivitis had poorer QoL; children referring "moderate/high" impact of rhinitis alone on daily activities were 4.6% compared to 10.7% of subjects with rhinoconjunctivitis (P=0.002, χ2 test). In a logistic model, when correcting for confounders, having conjunctivitis doubled the risk of "moderate/high" impact on QoL (Table 8).
In this cross-sectional evaluation, we identified an elevated prevalence of rhinitis and rhinoconjunctivitis among adolescents. The association of conjunctivitis with rhinitis (i.e., rhinoconjunctivitis) was more frequent in subjects with multiple allergic sensitizations and those exposed to environmental risk factors. The association of conjunctivitis with rhinitis strongly increased the risk for current asthma and had a significant impact on QoL.
Respiratory symptoms were investigated using the well-validated questionnaire-based definitions of the ISAAC Study.11 The core questions on rhinitis were highly specific, even though they showed a lower sensitivity for detecting atopy in a general population of children15. To address this issue, individual allergic sensitization was objectively evaluated using SPT. In our population sample, the prevalence of rhinitis (not associated with conjunctivitis) was 18.2%, while rhinoconjunctivitis prevalence was 20.5%; i.e., 38.7% of subjects had rhinitis in the last 12 months with or without conjunctivitis, and conjunctivitis was present in 52.9% of children with rhinitis. These results are comparable to those found in 2002 in Italian metropolitan areas following the same rhinitis and rhinoconjunctivitis definitions; conjunctivitis was reported in 56.6% of children with rhinitis.8 A survey conducted on 396 Swedish schoolchildren showed a prevalence of 17.6% for rhinoconjunctivitis and observed a comorbidity between rhinitis and conjunctivitis of ~92%.16 According to the ISAAC-phase III study, the prevalence of nasal symptoms associated with itchy/watery eyes ranged from 2.2%-24.2% in children and 4.5%-45.1% in adolescents.3 Determination of the prevalence of conjunctivitis by alone was not possible in the present study; in fact, the adopted questionnaire evaluated conjunctivitis only as a comorbidity of rhinitis.11 This did not allow the assessment of individuals possibly having conjunctivitis without symptoms of rhinitis.
By analyzing risk factors for the association between rhinitis and rhinoconjunctivitis, we excluded subjects with current asthma, which was more frequent in rhinitis and rhinoconjunctivitis. Since current asthma was strongly associated with rhinitis, the exclusion of current asthma from the analysis allowed better evaluation of the factors influencing the prevalence of rhinitis and rhinoconjunctivitis, in so far as asthma is linked to somewhat different risk factors than rhinitis.8 As reported in previous studies,17 rhinoconjunctivitis was associated with an increased frequency of parental history of atopy with respect to rhinitis, suggesting that a family history of allergic disease represents a risk factor for rhinoconjunctivitis in children. Similarly, rhinoconjunctivitis was associated with a higher level of allergic sensitization (i.e., higher AI) than rhinitis. After allergen stratification, sensitization to perennial allergens yielded an increased risk for rhinoconjunctivitis; a further increase was observed in subjects sensitized to both perennial and seasonal allergens. Conversely, sensitization to seasonal allergens resulted in no increase in conjunctivitis with respect to non-sensitized individuals. These observations are in line with the study of Weinmayr et al., suggesting that atopy is an important risk factor for rhinoconjunctivitis but only marginally relevant for rhinitis without conjunctivitis.18 Similarly, in a recent survey conducted in Norway, children with rhinitis and sensitization to at least one inhalant allergen had more frequent conjunctivitis than children with rhinitis without sensitization.19 Conversely, in a recent report on 9-11-year-old Turkish schoolchildren, atopy was not shown as a risk factor for rhinoconjunctivitis in children17, and Canonica et al. reported that ocular symptoms were more common in subjects with seasonal and seasonal plus perennial rhinitis than in those with perennial rhinitis.20
The ISAAC questionnaire also provided information on possible confounding factors and effect modifiers. In particular, self-reported exposure to traffic could be affected by reporting bias altering the association between reported exposure to road traffic and disease.21 Nevertheless, Nuvolone et al. demonstrated that a strong relationship exists between self-reported exposure to vehicular traffic and residential proximity to main roads, as evaluated using the Geographical Information System technology.22 In our bivariate models, conjunctivitis was significantly associated with rhinitis among subjects exposed to mold/dampness, ETS, and truck traffic. In a multiple logistic model, these variables were independent risk factors for having conjunctivitis associated with rhinitis. As shown previously,8,17 these results emphasize the role of "preventable/modifiable" factors on rhinoconjunctivitis. Mold exposure or sensitization was associated with rhinitis in clinical observations and epidemiological surveys,23 in particular during the first year of life.17
Studies examining the association between passive smoking and rhinoconjunctivitis are conflicting. Mitchell et al. found a weak association between ETS and symptoms of rhinoconjunctivitis, concluding that the relationship may not be causal.24 One study reported a reduced risk of rhinoconjunctivitis for children exposed to parental smoking.25 Conversely, Gonzalez-Diaz et al. showed that passive smoking is one of the risk factors for both rhinitis and rhinoconjunctivitis.26 More recently, we found that ETS is a significant risk factor for rhinoconjunctivitis.8 Similarly, a study by Montefort et al. reported that smoking by the mother alone was more common in children having current rhinitis, while smoking by the mother and/or father led to rhinoconjunctivitis.27
We also found that an increased frequency of high reported truck traffic in the residential streets was an independent risk factor for the association between rhinitis and conjunctivitis (OR 1.57). Although evidence exists that allergic respiratory disorders are associated with traffic exposure in children, the findings are not consistent. Many studies have found an association between traffic exposure and atopic sensitization,28,29,30 and there is experimental evidence that diesel particles may enhance allergic sensitization to common inhalant allergens.31 In agreement with these findings, an association was found between increased exposure to self-reported truck traffic on the residential streets and childhood symptoms of rhinoconjunctivitis.32 Similarly, Zuraimi et al. reported a correlation between self-reported traffic density and rhinoconjunctivitis.33 Conversely, a recent study found little or no association between ambient particulate pollution and the global rhinoconjunctivitis prevalence.34
Epidemiological and clinical data from many studies showed a significant association between rhinitis and asthma, supporting the hypothesis that rhinitis is a risk factor for asthma.4 Children with allergic rhinitis and asthma require a larger amount of medication than children with asthma alone, corroborating the hypothesis that the association between rhinitis and current asthma is characterized by increased disease severity.35 In the overall population sample, we found that rhinoconjunctivitis was more strongly associated with current asthma than rhinitis alone. In addition, using a logistics model, the presence of conjunctivitis significantly increased the risk for current asthma with respect to rhinitis independently of allergic sensitization. In sensitized children with rhinoconjunctivitis, the prevalence of current asthma increased to 17.3%; i.e., more than fourfold the current asthma prevalence in the overall sample (4.2%). Finally, rhinoconjunctivitis was associated with an increased number of visits to the hospital emergency department for asthma exacerbation, suggesting that the presence of conjunctivitis may be coupled with more severe disease. These results highlight the importance of conjunctivitis as an independent risk factor for asthma, supporting the link between upper and lower respiratory tracts (united airway disease). In this context, conjunctivitis should be considered part of this entity because the allergic inflammatory response involves the conjunctiva as well as the respiratory tract.3 In agreement with our results, recent studies have highlighted the significance of ocular symptoms, but few studies have explored the impact of allergic conjunctivitis as a comorbidity of asthma.3
Concerning the impact of rhinoconjunctivitis on QoL, we found that adolescents with rhinoconjunctivitis had significantly poorer QoL; in fact, 4.6% of subjects reported a high/moderate impact of rhinitis while 10.7% reported a high impact of rhinoconjunctivitis. Rhinoconjunctivitis is known to have an important impact on QoL, social interactions, and productivity both in children17 and adults.36 Ocular symptoms associated with allergic rhinitis, often under-diagnosed and under-treated, are increasingly recognized as a distinct disorder that imposes its own burden on medical costs and patient's QoL, especially in those with persistent moderate/severe nose symptoms.4 Approximately 50% of patients with rhinitis stated that ocular symptoms were moderately to extremely bothersome in the recent "Allergies in America" survey, and 10% of those complained that red/itching eyes were the most annoying symptom.37
In conclusion, our study investigated the impact of conjunctivitis on rhinitis and current asthma, as well as the risk factors and burden of rhinoconjunctivitis in schoolchildren. We found that atopic parental history, allergic sensitization, and environmental factors such as exposure to mold/dampness, ETS and heavy traffic are important risk factors for rhinoconjunctivitis. We also found that rhinoconjunctivitis is more strongly associated with current asthma than rhinitis and that the presence of conjunctivitis increases the risk for current asthma with respect to rhinitis alone. Finally, the presence of conjunctivitis may significantly worsen the QoL in patients with rhinitis. Since allergic ocular symptoms are often underdiagnosed and undertreated, clinicians should investigate these disorders and the risk factors within routine clinical practice to reduce the burden of disease in allergic patients, improving their QoL.
Figures and Tables
Table 1
General sample characteristics
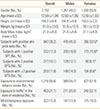
Table 2
Frequency distribution of subjects without rhinitis or rhinoconjunctivitis, subjects with rhinitis, and subjects with rhinoconjunctivitis with respect to the presence/absence of current asthma

Table 3
Effect of personal and environmental characteristics on the prevalence of rhinitis and rhinoconjunctivitis
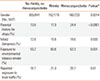
Table 4
Frequency distribution of subjects without rhinitis or rhinoconjunctivitis, subjects with rhinitis, and subjects with rhinoconjunctivitis for each class of Atopic Index (0-no allergic sensitizations, 1-one positive skin test, 2-two positive skin tests, 3-three or more positive skin tests). Differences were significant (P < 0.0001, χ2 test)

| Atopic index | No rhinitis, no rhinoconjunctivitis | Rhinitis | Rhinoconjunctivitis |
|---|---|---|---|
| Class 0 (%) | 66.3 | 16.6 | 17.1 |
| Class 1 (%) | 61.7 | 20.4 | 17.9 |
| Class 2 (%) | 57.8 | 19.1 | 23.1 |
| Class 3 (%) | 49.8 | 22.0 | 28.2 |
Table 5
Multiple logistic regression analysis of determinants for having conjunctivitis associated with rhinitis. Data are expressed as odds ratios (OR) and 95% confidence intervals (95% CI)

Table 6
Multiple logistic regression analysis of determinants for current asthma. Data are expressed as odds ratios (OR) and 95% confidence intervals (95% CI)

Table 7
Effects of different combinations of allergic sensitization and disease status (presence of rhinitis/rhinoconjunctivitis) on current asthma prevalence
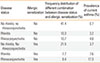
Table 8
Multiple logistic regression analysis for "moderate/high" rhinitis-related impact on quality of life. Data are expressed as odds ratios (OR) and 95% confidence intervals (95%CI)

*Overweight-obese children were defined following the gender- and age-specific cut-off points by Cole et al.13
ACKNOWLEDGMENTS
This research was supported in part by a grant from the Regional Agency for Environment Protection (ARPA Sicilia) [DDG No. 303/2005].
References
1. Björkstén B, Clayton T, Ellwood P, Stewart A, Strachan D. ISAAC Phase III Study Group. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008; 19:110–124.
2. Katelaris CH, Linneberg A, Magnan A, Thomas WR, Wardlaw AJ, Wark P. Developments in the field of allergy in 2010 through the eyes of clinical and experimental allergy. Clin Exp Allergy. 2011; 41:1690–1710.
3. Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011; 11:471–476.
4. Bielory L. Allergic conjunctivitis and the impact of allergic rhinitis. Curr Allergy Asthma Rep. 2010; 10:122–134.
5. Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001; 1:7–13.
6. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. World Health Organization. GA(2)LEN. AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.
7. Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet]. Vancouver (WA): Global Initiative for Asthma;2012. cited 2013 Dec 30. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_2012Feb13.pdf.
8. Cibella F, Cuttitta G, La Grutta S, Melis MR, Lospalluti ML, Uasuf CG, Bucchieri S, Viegi G. Proportional Venn diagram and determinants of allergic respiratory diseases in Italian adolescents. Pediatr Allergy Immunol. 2011; 22:60–68.
9. Galassi C, De Sario M, Biggeri A, Bisanti L, Chellini E, Ciccone G, Petronio MG, Piffer S, Sestini P, Rusconi F, Viegi G, Forastiere F. Changes in prevalence of asthma and allergies among children and adolescents in Italy: 1994-2002. Pediatrics. 2006; 117:34–42.
10. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998; 351:1225–1232.
11. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, Strachan F, Weiland SK, Williams HC. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995; 8:483–491.
12. Melki IS, Beydoun HA, Khogali M, Tamim H, Yunis KA. National Collaborative Perinatal Neonatal Network (NCPNN). Household crowding index: a correlate of socioeconomic status and inter-pregnancy spacing in an urban setting. J Epidemiol Community Health. 2004; 58:476–480.
13. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000; 320:1240–1243.
14. The European Academy of Allergology and Clinical Immunology. Position paper: allergen standardization and skin tests. Allergy. 1993; 48:48–82.
15. Braun-Fahrländer C, Wüthrich B, Gassner M, Grize L, Sennhauser FH, Varonier HS, Vuille JC. Validation of a rhinitis symptom questionnaire (ISAAC core questions) in a population of Swiss school-children visiting the school health services. SCARPOL-team. Swiss study on childhood allergy and respiratory symptom with respect to air pollution and climate. International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 1997; 8:75–82.
16. Hesselmar B, Aberg B, Eriksson B, Aberg N. Allergic rhinoconjunctivitis, eczema, and sensitization in two areas with differing climates. Pediatr Allergy Immunol. 2001; 12:208–215.
17. Civelek E, Yavuz ST, Boz AB, Orhan F, Yuksel H, Uner A, Cakir B, Sekerel BE. Epidemiology and burden of rhinitis and rhinoconjunctivitis in 9- to 11-year-old children. Am J Rhinol Allergy. 2010; 24:364–370.
18. Weinmayr G, Forastiere F, Weiland SK, Rzehak P, Abramidze T, Annesi-Maesano I, Björkstén B, Brunekreef B, Büchele G, Cookson WO, von Mutius E, Pistelli R, Strachan DP. ISAAC Phase Two Study Group. International variation in prevalence of rhinitis and its relationship with sensitisation to perennial and seasonal allergens. Eur Respir J. 2008; 32:1250–1261.
19. Bertelsen RJ, Carlsen KC, Carlsen KH. Rhinitis in children: co-morbidities and phenotypes. Pediatr Allergy Immunol. 2010; 21:612–622.
20. Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007; 62:Suppl 85. 17–25.
21. Kuehni CE, Strippoli MP, Zwahlen M, Silverman M. Association between reported exposure to road traffic and respiratory symptoms in children: evidence of bias. Int J Epidemiol. 2006; 35:779–786.
22. Nuvolone D, Della Maggiore R, Maio S, Fresco R, Baldacci S, Carrozzi L, Pistelli F, Viegi G. Geographical information system and environmental epidemiology: a cross-sectional spatial analysis of the effects of traffic-related air pollution on population respiratory health. Environ Health. 2011; 10:12.
23. Simoni M, Lombardi E, Berti G, Rusconi F, La Grutta S, Piffer S, Petronio MG, Galassi C, Forastiere F, Viegi G. SIDRIA-2 Collaborative Group. Mould/dampness exposure at home is associated with respiratory disorders in Italian children and adolescents: the SIDRIA-2 Study. Occup Environ Med. 2005; 62:616–622.
24. Mitchell EA, Beasley R, Keil U, Montefort S, Odhiambo J. ISAAC Phase Three Study Group. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from Phase Three of the ISAAC programme. Thorax. 2012; 67:941–949.
25. Hjern A, Hedberg A, Haglund B, Rosén M. Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents. Clin Exp Allergy. 2001; 31:908–914.
26. González-Díaz SN, Del Río-Navarro BE, Pietropaolo-Cienfuegos DR, Escalante-Domínguez AJ, García-Almaraz RG, Mérida-Palacio V, Berber A. Factors associated with allergic rhinitis in children and adolescents from northern Mexico: International Study of Asthma and Allergies in Childhood Phase IIIB. Allergy Asthma Proc. 2010; 31:e53–e62.
27. Montefort S, Ellul P, Montefort M, Caruana S, Grech V, Agius Muscat H. The effect of cigarette smoking on allergic conditions in Maltese children (ISAAC). Pediatr Allergy Immunol. 2012; 23:472–478.
28. Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, Kerkhof M, Brunekreef B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007; 29:879–888.
29. Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krámer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J. GINI Study Group. LISA Study Group. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008; 177:1331–1337.
30. Nordling E, Berglind N, Melén E, Emenius G, Hallberg J, Nyberg F, Pershagen G, Svartengren M, Wickman M, Bellander T. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology. 2008; 19:401–408.
31. Diaz-Sanchez D. The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease. Allergy. 1997; 52:52–56.
32. Brunekreef B, Stewart AW, Anderson HR, Lai CK, Strachan DP, Pearce N. ISAAC Phase 3 Study Group. Self-reported truck traffic on the street of residence and symptoms of asthma and allergic disease: a global relationship in ISAAC phase 3. Environ Health Perspect. 2009; 117:1791–1798.
33. Zuraimi MS, Tham KW, Chew FT, Ooi PL, Koh D. Home air-conditioning, traffic exposure, and asthma and allergic symptoms among preschool children. Pediatr Allergy Immunol. 2011; 22:e112–e118.
34. Anderson HR, Ruggles R, Pandey KD, Kapetanakis V, Brunekreef B, Lai CK, Strachan DP, Weiland SK. ISAAC Phase One Study Group. Ambient particulate pollution and the world-wide prevalence of asthma, rhinoconjunctivitis and eczema in children: Phase One of the International Study of Asthma and Allergies in Childhood (ISAAC). Occup Environ Med. 2010; 67:293–300.
35. Brito Rde C, da Silva GA, Motta ME, Brito MC. The association of rhinoconjunctivitis and asthma symptoms in adolescents. Rev Port Pneumol. 2009; 15:613–628.
36. Klossek JM, Annesi-Maesano I, Pribil C, Didier A. The burden associated with ocular symptoms in allergic rhinitis. Int Arch Allergy Immunol. 2012; 158:411–417.
37. Schulman, Ronca & Bucuvalas, Inc (US). Allergies in American kids: comparisons of children aged 4 to 17 with and without allergies [Internet]. New York (NY): Schulman, Ronca & Bucuvalas, Inc;2007. 04. cited 2013 Dec 30. Available from: http://www.worldallergy.org/allergic_rhinitis/resources/surveyfindings/.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download