Abstract
Purpose
House-dust-mite (HDM) major allergen Der p2 shares homology and function with Toll-like receptor (TLR) signaling protein myeloid differentiation-2 (MD2) and may lead to airway inflammation. Should Der p2 be internalized by human airway epithelium, it has the theoretical propensity to potentiate epithelium activation. This study aimed to demonstrate the internalization of Der p2 by airway epithelium and to investigate the effects of Der p2 on MD2 expression and epithelium activation.
Methods
Internalization of recombinant, enhanced green fluorescent protein-labelled Der p2 (rDer p2-EGFP) into human airway epithelium (BEAS-2B) was tracked by laser confocal microscopy and confirmed by immunoblotting. Reverse-transcription polymerase chain reaction (RT-PCR), immunoblotting, and immunohistochemical staining were used to determine the effect of Der p2 on MD2 expression in vitro and ex vivo. Expression of messenger RNA (mRNA) encoding receptors/cytokines was measured by RT-PCR. Secretion of interleukin-6/interleukin-8 (IL-6/IL-8) was measured by enzyme-linked immunosorbent assay (ELISA).
Results
Internalization of Der p2 by BEAS-2B was confirmed by confocal microscopy and immunoblotting using rDer p2-EGFP and rDer p2, respectively. Expression of MD2 protein was increased in BEAS-2B and human nasal polyp airway epithelium cultured with rDer p2. Recombinant Der p2-cultured BEAS-2B caused little spontaneous IL-6/IL-8 secretion but significantly augmented by TLR ligand LPS. IL-6 secretion was up-regulated after MD2 transfection. Internalization of Der p2 was reduced by TLR2 RNA knockdown. Dexamethasone, calcitriol, anti-MD2/anti-TLR2 antibodies, and signalling inhibitors significantly reduced LPS+Der p2-induced IL-6/IL-8 secretion.
Inhaled environmental allergens cause allergic symptoms in millions of patients worldwide. House dust mites have been identified as a common indoor allergen in many areas of the world, and exposure to mite allergens is a critical factor for the development of airway hypersensitivity.1 Our previous studies have confirmed that Dermatophagoides pteronyssinus (Der p) is a very common sensitizing allergen in patients suffering from atopic disease.2,3 Moreover, sensitization to Der p2, one of the major allergens of Der p, was implicated in 87.8% of these patients, particularly those under 10 years of age in Taiwan. Because of its importance, mechanisms of cellular activation by Der p2 have been studied in a variety of target cells.4,5,6
Myeloid differentiation-2 (MD2), is an LPS-binding member of the Toll-like receptor 4 (TLR4) signaling complex. Structures of MD2 and Der p2 exhibit homology, and Trompette et al.4 have reported that Der p2 can facilitate TLR4 signaling through direct interactions with the TLR4 complex, reconstituting LPS-promoted TLR4 signaling in the absence of MD2 and facilitating such signaling in the presence of MD2. In airway smooth muscle (ASM) cells, Der p2 also induces a high degree of proinflammatory cytokine expression by activating the MyD88 signaling pathway through TLR2.5 Since MD2 is a critical signaling molecule for a variety of inflammatory disorders and only trivially expressed in human epithelium in a basal state, its role in airway inflammation remains unclear.7 We have recently reported that Der p2 can up-regulate MD2 expression in B cells through activation of TLR4/MAPK (mitogen-activated protein kinase).8 It is therefore possible that Der p2 could also regulate MD2 expression in epithelium.
Prevention and treatment of Der p2-induced allergic airway inflammation have been intensively investigated as a means for hindering airway hypersensitivity induced by Der p2.9 Local nasal immunotherapy is a well-established procedure with Der p crude extract to down-regulate Der p2-triggered histamine release.10 It is therefore interesting to investigate a possible direct effect of Der p2 on epithelial activation.
So far, the direct evidence of internalization and activation of epithelium by Der p2 with direct imaging has never been obtained. Here, we aimed to use recombinant Der p2-EGFP (rDer p2-EGFP)11 to examine whether Der p2 could be internalized by human bronchial epithelium. Finally, we investigated the mechanisms for Der p2-induced epithelial activation and the potential anti-inflammatory effects of TLR/MAPK/NFκB pathway signaling inhibitors, corticosteroids, and calcitriol, an active form of vitamin D, which has recently been reported to exert a range of immunomodulatory activities and postulated to play key roles in the regulation of innate immunity.12,13,14
Neutralizing antibodies against human TLR2 and TLR4 were purchased from eBioscience (San Diego, CA, USA). Anti-MD2, anti-TLR2, anti-TLR4, and isotype control IgG antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The signaling inhibitors SP600125 (JNK inhibitor), SB203580 (p38 inhibitor), PD98059 (ERK inhibitor), and BAY 11-7082 (IκB inhibitor) were purchased from Calbiochem (Merck, Germany). LPS (Escherichia coli serotype 0111:B4), dexamethasone, and calcitriol were obtained from Sigma-Aldrich (St Louis, MO, USA). Recombinant-enhanced green fluorescent protein (rEGFP) was purchased from BioVision (Mountain View, CA, USA).
Recombinant Der p2 (rDer p2) construct was transformed into Pichia pastoris (Invitrogen, Carlsbad, CA, USA). Secreted-rDer p2 supernatant was harvested and concentrated. Recombinant Der p2-EGFP (rDer p2-EGFP) was expressed in Escherichia coli M15. Expression of rDer p2-EGFP was performed using the QIAexpressionist kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Isopropyl-β-D-thiogalactopyranoside-induced 6xHis-tagged recombinant protein in inclusion bodies was solubilized with 8 M urea and purified under native conditions using nickel-nitrilotriacetic acid affinity column chromatography (Novagen, Madison, WI, USA). Purified rDer p2-EGFP was further clarified by Endotoxin Detoxi-Gel (Thermo, Wilmington, DE, USA) to remove LPS. Residual LPS was measured using the ToxinSensor Chromogenic LAL Endotoxin Assay kit (GenScript, Piscataway, NJ, USA). The purities of rDer p2 and rDer p2-EGFP were analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie Brilliant Blue R-250 staining and their identities confirmed by specific antibodies (data not shown).
The human bronchial epithelial cell line BEAS-2B purchased from the American Type Culture Collection was maintained in RPMI-1640 culture medium supplemented with 10% (v/v) heat-inactivated FBS (HyClone, Logan, UT, USA). Before experimental treatments, plated cells were starved in serum-free medium for 24 hours. For inhibition studies, cells were pretreated with various inhibitors in serum-free medium for 2 hours prior to LPS and/or rDer p2 stimulation.
BEAS-2B cells were plated in 8-well or 6-channel ibiTreat µ-slide chambers (ibidi GmbH, Martinsried, Germany), starved and treated with rEGFP or rDer p2-EGFP (5 µg/mL), and transduced with CellLight™ endoplasmic reticulum-red fluorescent protein (ER-RFP) (Invitrogen). Fluorescence in living cells was traced and recorded using an Olympus FV1000 confocal laser scanning biological microscope.
BEAS-2B cells were pretreated with various inhibitors or medium control, and then stimulated with rDer p2 in conjunction with or without LPS. Total RNA extraction was performed using a TRIZOL reagent protocol (Invitrogen). First-strand cDNA was synthesized from 1 µg of total RNA by reverse transcription in a 10 µL reaction using a SuperScrip III first-strand cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. Expression of mRNA encoding MD2, TLR2, TLR4, IL-1β, IL-6, and IL-8 was analyzed by RT-PCR. All PCR assays were performed using PCR master mix. Primers used for PCR analyses were listed in Table. A suitable temperature cycle profile for each PCR reaction was obtained using an MJ Mini Personal Thermal Cycler (Bio-Rad, Foster, CA, USA). The intensities of DNA bands in agarose gel were quantified using a Gel-Pro Analyzer (Media Cybernetics, Inc., MD, USA) and normalized to β-actin.
After BEAS-2B cells were incubated with rDer p2 (10 µg/mL) for 24 hours, cellular proteins of Der p2, MD2, and β-actin were separated by SDS-PAGE and identified by immunoblotting. The antigen-antibody complex was detected with ECL reagents (Millipore, Darmstadt, Germany), followed by exposure to X-ray films.
Nasal polyps were obtained at routine polypectomy from patients who visited the clinics of Otolaryngology in Taichung Veterans General Hospital (TCVGH), Taichung, Taiwan. The study was approved by the Institutional Review Board of TCVGH (TCVGH IRB No. C06001). Informed consents were obtained from all the study patients and participants. Nasal polyps were incubated with medium or rDer p2 (10 µg/mL) for 4 hours, fixed with paraformaldehyde (PFA), and embedded in paraffin. The paraffin-embedded sections were stained with anti-MD2 antibody using a modified peroxidase-based method as previously described.15,16 MD2 antibody immunostained sections were visualized, and images were acquired using a Leica microscope. The mean intensity of DAB staining was measured using TissueGnostics (TissueGnostics Ltd., Mountain view, CA, USA).
BEAS-2B cells were pretreated with various inhibitors as presented in the Results section. LPS and rDer p2 were then introduced to the cells and incubated for further 24 hours. At the end of incubation, cell-free culture supernatants were harvested for proinflammatory cytokines (IL-6/IL-8) measurement using DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Absorbance was measured with a microplate reader at a wavelength of 450/620 nm.
Total RNA was isolated from BEAS-2B cells, and then reverse transcribed into cDNA. RT-PCR was performed for MD2 gene amplification with the following primers (EcoRI restriction sites are underlined): forward primer 5'-CGG AAT TCA TGT TAC CAT TTC TGT TTT TTT CCA CCC TG-3'; reverse primer 5'-CGG AAT TCC TAA TTT GAA TTA GGT TGG TGT AGG ATG AC-3'. PCR product was digested with EcoRI restriction enzyme and subcloned into the corresponding site of the pcDNA3.1 plasmid. BEAS-2B cells were plated onto a 6-well plate at 1×106 cells/well, grown overnight, and transiently transfected for 24 hours with the pcDNA-MD2 plasmid using the DharmaFECT transfection reagent (Thermo). Cells were recovered for 24 hours and checked for MD2 overexpression by immunoblotting. Transiently transfected cells were stimulated with medium or rDer p2 (5 µg/mL) for another 24 hours, cell-free culture supernatants were harvested for IL-6 measurement by ELISA, and cells were analyzed for MD2 protein expression with immunoblotting.
To achieve TLR2 RNA knockdown, the vector expressing shTLR2 was obtained from National RNAi Core Lab of the Academia Sinica and transiently transfected into BEAS-2B cells. The target sequence of shTLR2 is 5'-GCGGAAGATAATGAACACCAA-3'. Transiently transfected cells were stimulated with medium or rDer p2 (10 µg/mL) for 24 hours, followed by intracellular Der p2 protein analysis by immunoblotting.
Data are expressed as mean±SD. Statistical analysis was performed using Student's t test and one-way analysis of variance (ANOVA). Differences were considered statistically significant at *P<0.05, **P<0.01, or ***P<0.001. Data were obtained from at least 3 independent experiments.
BEAS-2B monolayers were cultured with rDer p2-EGFP or rEGFP alone. Fluorescent images of living cells were obtained by confocal microscopy. Der p2-EGFP was observed in the cellular cytoplasm following 4 hours (Fig. 1B) and 24 hours (Fig. 1C) of incubation. There was no fluorescence in the cytoplasm of cells incubated with rEGFP alone (Fig. 1A); instead the fluorescent label was collected around the cellular margins, delineating the shapes of the cells, but disappeared with washing. By contrast, rDer p2-EGFP-treated cells retained fluorescence in the cytoplasm after washing (data not shown). When cells were cultured with rDer p2-EGFP and ER-RFP, internalized Der p2 in the cytoplasm co-localized with ER following 24 hours of incubation (Fig. 1C).
BEAS-2B cells were collected for mRNA and protein analysis following incubation with rDer p2 or medium control. Expression of mRNA-encoding MD2 was identified in the lysed cells by RT-PCR after 4 hours of culture, but this required 2 consecutive rounds of PCR amplification, following which its expression was increased in cells treated with rDer p2 as compared to medium control (Fig. 2A). Both Der p2 and MD2 proteins were identified in the cellular lysates by immunoblotting with anti-Der p2 and anti-MD2 antibodies following 24 hours of incubation. The expression of both Der p2 and MD2 was markedly elevated in the epithelium incubated with rDer p2 compared to medium control (Fig. 2B). Nasal polyps from patients with HDM allergy were cultured with medium only or rDer p2 for 4 hours, followed by image analysis of MD2 expression in the epithelium. MD2 was identified in nasal epithelium (Fig. 2C), and its expression was increased following incubation with rDer p2 compared to medium control (Fig. 2D).
BEAS-2B cells were cultured with rDer p2-EGFP with/without additional LPS for 24 hours. Supernatants were collected for IL-6/IL-8 measurement. Although rDer p2-EGFP alone induced trivial secretion of IL-6/IL-8 by BEAS-2B cells, it was concentration-dependently augmented by LPS from 2.0- to 19.1/78.8-fold for IL-6 and from 3.3- to 31.7/104.9-fold for IL-8, respectively, in comparison to medium controls (Fig. 3A). We investigated the effect of anti-MD2 antibody on this phenomenon. Cells were cultured with/without anti-MD2 antibody and rDer p2 in conjunction with LPS for 24 hours, and then culture supernatants were collected for IL-6/IL-8 measurement. IL-6/IL-8 secretion was reduced in the presence of anti-MD2 antibody from 25.7- to 11.5-fold for IL-6 and from 44.6- to 28.5-fold for IL-8, respectively, in comparison to medium control (Fig. 3B). Cells were transiently transfected with MD2 to induce overexpression which was confirmed by immunoblotting with anti-MD2 antibody (Fig. 4A). Compared to non-transfected cells, cells overexpressing MD2 were more sensitive to rDer p2 stimulation for IL-6 secretion (Fig. 4B).
BEAS-2B cells were cultured with 2 concentrations of rDer p2 with/without LPS for various time periods (3, 6, and 16 hours), then the cells were lysed for mRNA extraction. Der p2 concentration-dependently increased the expression of mRNA encoding IL-6 and IL-8 in these cells over the 16-hours time period, and this effect was further augmented in the presence of LPS. In contrast, there was little or no change in expression of mRNA encoding TLR4, whereas the mRNA expression of TLR2 and IL-1β was somewhat increased, especially in conjunction with LPS (Fig. 5A and B). Cells were also cultured with/without rDer p2 in conjunction with LPS for 6 hours in the presence of various inhibitors as described in the Results section. Of these, the most effective inhibitors on IL-6 mRNA expression were dexamethasone, SP600125 (JNK inhibitor), SB203580 (p38 inhibitor), calcitriol, and BAY 11-7082 (IκB inhibitor), which caused 77.0%, 65.5%, 33.8%, 33.8%, and 25.0% reductions, respectively, in the fold induction of IL-6 mRNA induced by rDer p2 in conjunction with LPS (Fig. 6A and B). The most effective inhibitors on IL-8 mRNA expression were dexamethasone, SP600125, calcitriol, and BAY 11-7082, which caused 42.4%, 30.0%, 27.3%, and 24.2% reductions, respectively, in the fold induction of IL-8 mRNA induced by rDer p2 in conjunction with LPS (Fig. 6A and B). A relatively high concentration of Der p2 protein was detected in the cellular lysates of rDer p2-treated BEAS-2B cells. This was notably reduced in shTLR2-knockdowned cells (Fig. 6C).
When BEAS-2B cells were cultured with rDer p2 in conjunction with LPS, IL-6/IL-8 proteins were measured in the culture supernatants following 24 hours of incubation. All the transcription factor-signaling inhibitors reduced secretion of both IL-6 and IL-8 (Fig. 7A). Similar effects were observed in the presence of dexamethasone, calcitriol, and a neutralizing antibody against TLR2, but not against TLR4 (Fig. 7B and C). The most effective inhibitors on IL-6/IL-8 protein secretion were dexamethasone, SP600125 (JNK inhibitor), SB203580 (p38 inhibitor), anti-TLR2 neutralizing antibody, and calcitriol (Fig. 7D).
In this study, we confirmed, by confocal microscopy and immunoblotting, that human airway epithelium internalizes Der p2. Although this appeared to induce trivial cellular activation alone, at least in terms of the readouts we chose to study, it markedly increased the secretion of both IL-6 and IL-8 by these cells in response to rDer p2 in conjunction with LPS. The possible relevance of this phenomenon to the development of mucosal inflammation and permeability in diseases, such as asthma and chronic rhinosinusitis, and possibly host defense also deserves much more detailed exploration. In this study, Der p2 internalization increased endogenous cellular expression of MD2 and an anti-MD2 antibody significantly reduced the response of the cells to Der p2/LPS, while MD2 overexpression greatly enhanced IL-6 secretion in response to this stimulus. These results suggest a vital role for MD2 in Der p2/LPS induced airway epithelium activation, even though its baseline expression in these cells appeared to be very low. The phenomena of Der p2 internalization could increase cellular expression of MD2 and were also confirmed in nasal airway epithelium ex vivo. A previous study has also suggested a vital role of MD2 in mediating endotoxin responsiveness of epithelium.7
In our study, the reason anti-TLR2 but not anti-TLR4 inhibited Der p2/LPS-induced IL-6/IL-8 secretion would be the spectrum of ligand responsiveness to TLR2 and TLR4. While both TLR2 and TLR4 have been reported to function as LPS-signal transducers, they have different roles in pathogen recognition.17 In particular, the spectrum of responsiveness to TLR2 is wider than that to TLR4 because both receptors respond to LPS, but TLR2 additionally responds to lipoproteins, lipopeptides, and peptidoglycans.18
Although TLR4 expression can be up-regulated by LPS,4 it has also been reported that LPS can increase TLR2 expression in human respiratory epithelium, such as A549.19 Our finding reproduced with BEAS-2B cells in the presence of Der p2 supports the hypothesis that LPS/Der p2 stimulation synergistically enhances TLR2 expression. The fact that LPS does not appear further to increase TLR4 expression in BEAS-2B cells may simply reflect its initially high expression compared to TLR2 in these cells in the resting state.
In this study, cellular activation was clearly inhibited by TLR2-neutralizing antibody and MAPK inhibitors, indicating that the TLR2/MAPK pathway was involved. Epithelium activation by Der p2 in conjunction with LPS could be down-regulated by a JNK inhibitor (SP600125), SB203580 (p38 inhibitor), anti-TLR2-neutralizing antibody, and calcitriol in addition to anti-inflammatory corticosteroid dexamethasone. A similar finding was reported in mouse airway smooth muscle (ASM) cells, in which Der p2 was shown to activate ASM cells in a TLR2/MyD88-dependent manner.5 These findings suggest that TLR2/MAPK might play an important role in the pathogenesis of BEAS-2B activation by Der p2. Another previous study also supports this contention.20
To study the internalization of Der p2 by BEAS-2B cells, we used RNA interference of TLR2 with knockdown TLR2 expression. We observed that the knockdown of TLR2 indeed reduced the internalization level of Der p2, indicating that the ingress of Der p2 might be somehow dependent on TLR2 expression.
Xue et al.21 have demonstrated the potential for calcitriol, a biologically active form of vitamin D, to reduce Pseudomonas aeruginosa-induced expression of IL-6 and IL-8 by epithelium, suggesting its potential to become a novel anti-inflammatory drug.21 Rostkowska-Nadolska et al.22 have demonstrated the potential of calcitriol to reduce proinflammatory cytokine secretion by fibroblasts isolated from nasal polyps.22 We observed similar activity of calcitriol in this study.
A previous study has shown that native, but not recombinant, Der p2 which lacks glycosylated lectin ligands can stimulate monocyte-derived dendritic cells through its interaction with DC-SIGN, a lectin receptor.6 Our result indicating that recombinant, non-glycosylated Der p2 was internalized by epithelium suggests that this process may not be mediated through lectin receptors such as DC-SIGN, although we did not formally compare the internalization between native/glycosylated Der p2 and recombinant/non-glycosylated Der p2.
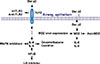
In summary, we demonstrated a novel mechanism for mite-facilitated airway epithelial inflammation induced by interactions between mite allergen and other environmental stimuli acting on innate immune receptors. These mechanisms offer a much deeper understanding of how mite allergens may initiate and exacerbate diseases, such as asthma and rhinosinusitis. As shown in Fig. 8, Der p2 could be internalized by human airway epithelium, and the activation of epithelium to secrete IL-6/IL-8 might up-regulate MD2 and TLR2. Secretion of IL-6/IL-8 was inhibited by MAPK and IκB inhibitors, suggesting that MAPK and NFκB signaling pathways may be involved in allergic airway inflammation. In addition to conventional pharmacotherapy with dexamethasone, calcitriol provided good anti-inflammatory effects which may have the potential to serve as an alternative anti-inflammatory agent.
Figures and Tables
Fig. 1
Images of Der p2-EGFP and co-localized with ER-RFP in BEAS-2B cells by confocal laser scanning biological microscopy. Cells were cultured with 5 µg/mL of rDer p2-EGFP or rEGFP alone. No fluorescence could be detected in cells incubated with rEGFP alone (A). Fluorescence from Der p2-EGFP appeared in the cytoplasm after 4-hour (B) and 24-hour (C) incubation. Cells were also co-transduced with CellLight™ ER-RFP. Der p2-EGFP co-localized with ER-RFP after 24-hour incubation (C). A representative image and an actual figure representative of the 3 independent experiments with similar findings are shown. DIC, differential interference contrast; EGFP, enhanced green fluorescent protein; ER-RFP, endoplasmic reticulum-red fluorescent protein.
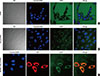
Fig. 2
Increased expression of MD2 mRNA and Der p2/MD2 protein in rDer p2-treated BEAS-2B cells and MD2 in nasal polyps. The expression of MD2 mRNA after 1 (1X) and 2 (2X) consecutive rounds of PCR amplification (4 hours) (A), Der p2 and MD2 protein by immunoblotting (24 hours) (B) in BEAS-2B cells incubated with 10 µg/mL of rDer p2. (C) Nasal polyps were cultured (4 hours) with medium only (left) or 10 µg/mL of rDer p2 (right). Tissue sections were immunostained with anti-MD2 antibody using DAB as a peroxidase substrate, and the mean intensity of staining was measured objectively (D). A representative image and an actual figure representative of the 3 independent experiments with similar findings are shown.
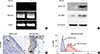
Fig. 3
Enhanced effects of LPS and reduced effects of anti-MD2 antibody on IL-6/IL-8 protein secretion by Der p2-activated BEAS-2B cells. Cells were cultured with rDer p2 in conjunction with LPS without (A) and with (B) additional anti-MD2 antibody for 24 hours. Fold changes in expression are shown in comparison to control treatment with medium. Data are presented as mean±SEM of the 3 separate experiments. A representative image and an actual figure representative of the 3 independent experiments with similar findings are shown. **P<0.01; ***P<0.001.

Fig. 4
MD2 overexpression increased IL-6 secretion by Der p2-activated BEAS-2B cells. MD2 overexpression was confirmed by immunoblotting with anti-MD2 antibody (A), the increasing fold of IL-6 secretion are shown in comparison to non-transfected control cells treated with medium (B). Data are presented as mean± SEM of the 3 separate experiments. A representative image and an actual figure representative of the 3 independent experiments with similar findings are shown.

Fig. 5
Effects of Der p2 with or without LPS on expression of mRNA encoding Toll-like receptors and cytokines in BEAS-2B cells cultured for 3, 6, and 16 hours. (A) Der p2 notably increased the expression of mRNA encoding IL-6 and IL-8, and this effect was further augmented in the presence of LPS. There was little or no change in the expression of mRNA encoding TLR4, whereas TLR2 and IL-1β were somewhat increased, especially in conjunction with LPS. Quantitative data on band densities were acquired by densitometric analysis and expressed relative to those of the β-actin loading control, which are shown under the bands. (B) Densitomeric analysis data are presented as the mean±SEM. A representative image and an actual figure representative of the 3 independent experiments with similar findings are shown.

Fig. 6
Effects of various inhibitory agents on mRNA expression in LPS + rDer p2-treated BEAS-2B cells and effects of TLR2 knockdown on the internalization of Der p2 by BEAS-2B cells. (A) Messenger RNA encoding TLR2, IL-6, and IL-8, and β-actin loading control in BEAS-2B cells cultured (6 hours) with rDer p2 in conjunction with LPS. Quantitative data were acquired by densitometric analysis and normalized to the expression of β-actin, which are shown under the bands. (B) Reduced fold induction of IL-6 and IL-8 mRNA induced by rDer p2 in conjunction with LPS. The most effective inhibitors on IL-6/IL-8 mRNA expression were dexamethasone, SP600125 (JNK inhibitor), calcitriol, SB203580 (p38 inhibitor), and BAY 11-7082 (IκB inhibitor). Reduction of fold induction (%) = [1- (LPS + rDer p2 stimulation with inhibitor/without inhibitor)] ×100%. Data are presented as the mean±SEM. (C) TLR2-knockdowned cells were incubated with rDer p2 for 24 hours, followed by rDer p2 protein analysis with immunoblotting. The concentration of Der p2 protein was notably reduced in the cellular lysates of shTLR2-knockdowned and rDer p2-treated cells. A representative image and an actual figure representative of the 3 independent experiments with similar findings are shown. *P<0.05; **P<0.01.

Fig. 7
Effects of various inhibitory agents on IL-6/IL-8 protein secretion in LPS + rDer p2-treated BEAS-2B cells. (A) Transcription-signalling inhibitors, (B) anti-inflammatory drugs and (C) neutralizing antibodies on IL-6/IL-8 protein secretion (24 hours) by LPS (50 ng/mL)- and rDer p2 (5 µg/mL)-stimulated BEAS-2B cells. (D) Reduction of fold induction by various agents on rDer p2 in conjunction with LPS-induced IL-6/IL-8 secretion. The most effective inhibitors on IL-6/IL-8 protein secretion were dexamethasone, SP600125 (JNK inhibitor), SB203580 (p38 inhibitor), anti-TLR2 neutralizing antibody, and calcitriol. Reduction of fold induction (%) = [1 - (LPS + rDer p2 stimulation with inhibitor/without inhibitor)] ×100%. Data presented as mean±SEM of 3 independent experiments. **P<0.01; ***P<0.001.

Fig. 8
Schematic diagram of Der p2 internalization and induction of human airway epithelial cell activation. Der p2 was internalized by epithelial cells, and the up-regulated MD2 and TLR2 resulted in IL-6/IL-8 secretion, which were confirmed by anti-MD2 antibody, anti-TLR2 antibody, and shTLR2. The secretion of IL-6/IL-8 was also inhibited by MAPK/IκB inhibitors, dexamethasone, and calcitriol.
Table
Primer sequences and sizes used in RT-PCR

ACKNOWLEDGMENTS
This research was supported by grants to Jaw-Ji Tsai from the Veterans General Hospitals University System of Taiwan Joint Research Program (VGHUST-100-G625) and Taichung Veterans General Hospital (TCVGH-1007318D).
We are grateful to the patients who participated in this study, to Chris Corrigan for critically reviewed this manuscript, to Pei-Jing Chen for providing technical support for immunohistochemical staining of nasal polyp epithelium, to Mei-Chun Liu for providing technical assistance with the confocal microscope, and to Hsing-Keng Tsai for providing secretary assistance.
References
1. Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997; 100:S2–24.
2. Tsai JJ, Kao MH, Huang SL. Comparison of major aeroallergens in Taipei and Kin-Men. J Formos Med Assoc. 1997; 96:985–989.
3. Tsai JJ, Shen HD, Chua KY. Purification of group 2 Dermatophagoides pteronyssinus allergen and prevalence of its specific IgE in asthmatics. Int Arch Allergy Immunol. 2000; 121:205–210.
4. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.
5. Chiou YL, Lin CY. Der p2 activates airway smooth muscle cells in a TLR2/MyD88-dependent manner to induce an inflammatory response. J Cell Physiol. 2009; 220:311–318.
6. Hsu SC, Chen CH, Tsai SH, Kawasaki H, Hung CH, Chu YT, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010; 285:7903–7910.
7. Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L428–L437.
8. Tsai JJ, Liu SH, Yin SC, Yang CN, Hsu HS, Chen WB, et al. Mite allergen Der-p2 triggers human B lymphocyte activation and Toll-like receptor-4 induction. PLoS One. 2011; 6:e23249.
9. Liu YH, Kao MC, Lai YL, Tsai JJ. Efficacy of local nasal immunotherapy for Dp2-induced airway inflammation in mice: using Dp2 peptide and fungal immunomodulatory peptide. J Allergy Clin Immunol. 2003; 112:301–310.
10. Liao EC, Tsai JJ. Clinical effectiveness of Tyrophagus putrescentiae allergy by local nasal immunotherapy using strips of Dermatophagoides pteronyssinus. J Asthma. 2011; 48:957–964.
11. Merkle D, Zheng D, Ohrt T, Crell K, Schwille P. Cellular dynamics of Ku: characterization and purification of Ku-eGFP. Chembiochem. 2008; 9:1251–1259.
12. Rostkowska-Nadolska B, Latocha M, Gawron W, Kutner A, Bochnia M. The influence of calcitriol and tacalcitol on proliferation of fibroblasts cultured from nasal polyps. Adv Clin Exp Med. 2007; 16:213–219.
13. Sato H, Ogino Y, Takagi H, Hata J, Asano S, Ohta T, et al. Pharmacological profiles of high-concentration (20 microg/g) tacalcitol ointment: effects on cutaneous inflammation, epidermal proliferation, and differentiation in mice. J Dermatol. 2003; 30:510–524.
14. Tukaj S, Trzonkowski P, Tukaj C. Regulatory effects of 1,25-dihydroxyvitamin D3 on vascular smooth muscle cells. Acta Biochim Pol. 2012; 59:395–400.
15. Clements D, Asprey SL, McCulloch TA, Morris TA, Watson SA, Johnson SR. Analysis of the oestrogen response in an angiomyolipoma derived xenograft model. Endocr Relat Cancer. 2009; 16:59–72.
16. Giaid A, Michel RP, Stewart DJ, Sheppard M, Corrin B, Hamid Q. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993; 341:1550–1554.
17. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999; 274:10689–10692.
18. Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999; 274:33419–33425.
19. Wu TT, Chen TL, Loon WS, Tai YT, Cherng YG, Chen RM. Lipopolysaccharide stimulates syntheses of toll-like receptor 2 and surfactant protein-A in human alveolar epithelial A549 cells through upregulating phosphorylation of MEK1 and ERK1/2 and sequential activation of NF-kappaB. Cytokine. 2011; 55:40–47.
20. Tanyaratsrisakul S, Jirapongsananuruk O, Thomas WR, Piboonpocanun S, Voelker DR. Der p2 stimulate inflammatory responses from lung epithelial cells and macrophages through the TLR2 and MAPK pathway. J Allergy Clin Immunol. 2012; 129:AB140.
21. Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002; 80:340–345.
22. Rostkowska-Nadolska B, Sliupkas-Dyrda E, Potyka J, Kusmierz D, Fraczek M, Krecicki T, et al. Vitamin D derivatives: calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv Med Sci. 2010; 55:86–92.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download