Abstract
Purpose
Divergent results on the IgE reactivity of dog-allergic subjects to Can f 4 have been reported. The aim of this study was to evaluate the significance of Can f 4 in dog allergy and to develop an immunochemical method for measuring Can f 4 content in environmental samples.
Methods
We purified the natural dog allergen Can f 4 from a dog dander extract by monoclonal antibody-based affinity chromatography and generated its variant in a recombinant form. Sixty-three dog-allergic patients and 12 nonallergic control subjects were recruited in the study. The IgE-binding capacity of natural Can f 4 and its recombinant variant was assessed by ELISA, immunoblotting, and skin prick tests (SPT).
Results
Eighty-one percent of the dog-allergic patients showed a positive result to the immunoaffinity-purified natural Can f 4 in IgE ELISA, but only 46% in IgE immunoblotting. Respective results with the recombinant Can f 4 variant were 54% and 49%. SPT results reflected those obtained in ELISA and immunoblotting. The overall IgE reactivity of the immunoaffinity-purified natural Can f 4 was found to depend strongly on the integrity of the allergen's conformation. A sandwich ELISA based on monoclonal antibodies was found to be functional for measuring Can f 4 in environmental samples.
Dog is a source of several important allergenic molecules. These allergens are found everywhere indoors, including public places1,2 and homes without dogs.1,3 It has been estimated that exposure to the dog allergen Can f 1 in schools or buses can reach levels able to cause allergic symptoms in sensitized subjects.4
For the time being, the number of officially recognized dog allergens is 6.5 Four of the allergens, Can f 1,6 Can f 2,6 Can f 4,7,8 and Can f 69 are lipocalins, whereas Can f 3 is serum albumin10 and Can f 5 is prostatic kallikrein.11 It is of interest that none of these allergens stands out as a superior sensitizer, as is the case with Bos d 2 in cow allergy12 or Fel d 1 in cat allergy.13 However, IgE reactivities up to 60%-70% have been reported to several of them, i.e., Can f 1,6 Can f 4,7 Can f 6,9 and Can f 5.11
The observed IgE reactivity in a population to an allergen can depend on several factors, such as the subjects included, the type of exposure, the allergen preparation, or the methods used. Probably therefore, IgE prevalences, for example, to Can f 1 have varied considerably from 42% to 75%.6,14 Based on IgE reactivity in immunoblotting, we previously reported that 60% of dog-allergic subjects had IgE to an 18 kDa allergen in dog dander, Can f 4.7 Later Mattsson et al.8 found, however, a substantially lower prevalence of IgE reactivity, 35%, using the ImmunoCAP method with recombinant (r) Can f 4.
In this work, we sought to clarify the discrepancy found in the dog-allergic subjects' IgE reactivity to Can f 4. For that purpose, we purified natural Can f 4 from a dog dander extract and generated a recombinant form of the allergen. Moreover, an immunochemical method was developed for assessing exposure to the allergen. We found that a recombinant variant of Can f 4, produced in Pichia pastoris, resembled the immunoaffinity-purified natural (n) Can f 4 in its IgE-binding capacity, although it exhibited a lower capacity to bind IgE than the natural allergen in ELISA. Importantly, the IgE binding capacity of immunoaffinity-purified natural Can f 4 was strongly affected by denaturation, which may be a factor accounting for the previously found conflicting results on the IgE reactivity to Can f 4. The developed two-site ELISA appears to be functional for measuring the Can f 4 content in environmental samples.
A total of 63 randomly selected dog-allergic subjects (35 women [20 Finnish and 15 Spanish], mean age 38.8±11.2 years(data were presented as mean±standard deviation, otherwise indicated), and 28 men [17 Finnish and 11 Spanish] mean age 39.3±9.8 years) presenting with allergic symptoms upon exposure to dog, together with 12 healthy nonatopic individuals as control subjects (9 women [Finnish], mean age 35.9±11.9 years, and 3 men [Finnish], mean age 28.7±1.5 years) were recruited to the study. A patient was classified as allergic to dog if the result of the dog dander UniCAP specific IgE measurement (Pharmacia, Uppsala, Sweden) was >0.35 kU/L (mean IgE response of the dog allergic cohort to dog epithelium extract was 12.5 kU/L [range; 0.38-100 kU/L]) or the result of the skin prick test (SPT) using commercial dog epithelial extract (ALK Abelló, Hørsholm, Denmark) was ≥3 mm (mean; 5.8 mm, range; 3-18 mm). SPTs against nCan f 4 and rCan f 4 were performed to 16 dog-allergic patients together with 6 control subjects (both groups were Finnish) according to European recommendations, as previously described.15,16 The study was approved by the Ethics Committee of the Kuopio University Hospital with the permit number 182/1999, valid until 2015.
Dog dander raw material (mixed breed RME64P, Greer laboratories, Lenoir, NC, USA) was extracted in PBS overnight at 4℃, essentially, as previously described,12 to prepare in-house dog allergen extract. A cocktail of 3 Can f 4 allergen-specific monoclonal antibodies (mAbs) (for the generation of mAbs 26D, 41G, and 48F, see Supplementary Material 1) was coupled to the HiTrap NHS column (GE Healthcare, Amersham Biosciences, Uppsala, Sweden), and the allergen was purified from the extract according to the manufacturer's instructions. The protein concentration of the allergen was determined by the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Inc., Hercules CA, USA) using bovine serum albumin as a standard. The purity of the allergen preparation was verified by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)7 with silver staining.17 For details, see Supplementary Material 1.
For mass spectrometric analysis and N-terminal sequencing, the purified natural Can f 4 allergen was, after desalting by reversed phase chromatography, subjected to MALDI-TOF mass spectrometry and Edman degradation. For further amino acid sequence determination, the protein was reduced, alkylated, and digested with trypsin, and the peptides were separated by reversed phase chromatography. Selected individual peptides were then subjected to MALDI-TOF mass spectrometry and sequencing by Edman degradation (Supplementary Material 1).
Carbohydrate moieties covalently linked to the nCan f 4 allergen backbone were examined with SDS-PAGE using the Glycoprotein Detection Kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer's instructions.
Molecular cloning of the DNA encoding the Can f 4 sequence into the Pichia pastoris yeast, the production of the recombinant allergen,18 and its purification in both monomeric and multimeric forms are described in Supplementary Material 1. Molecular mass and identity of the purified rCan f 4 was determined with ESI-Quad-TOF by direct infusion. The identity of rCan f 4 was confirmed by LC-MS/MS and subsequent Mascot database search, as previously described for details (Supplementary Materials 1 and 2).
The production and purification of other recombinant proteins of the lipocalin family, dog Can f 1/Can f 2, horse Equ c 1, mouse Mus m 1, cow Bos d 2, human tear lipocalin (TL; lipocalin-1/von Ebner's gland protein), and the recombinant control protein psoriasin have been previously described in our publications.7,14,19,20
Immunization of the BALB/c mice and hybridoma production are described in detail in Supplementary Material 1. In brief, supernatants of the generated hybridomas were tested for reactivity to the 18 kDa component of dog dander (that was later designated as Can f 4) with ELISA and immunoblotting. The antibodies 26D, 41G, and 48F were purified by affinity chromatography on a protein-G column (GE Healthcare) and the isotypes of the antibodies were determined by the Clonacell InstantCHEK One-minute Isotype Kit (Stem Cell, Vancouver, Canada). Protein concentrations were determined by the Bio-Rad Protein Assay Kit using bovine gamma globulin as a standard.
For assessing whether the 3 mAbs specific to Can f 4 recognize distinct epitopes on the allergen, they were first labeled with biotin. Biotinylation was carried out using aminohexanoyl-biotin-N-hydroxysuccinimide ester (AH-BNHS, Zymed Laboratories, Inc., San Francisco, CA, USA) at a 1:10 w/w ratio of AH-BNHS and mAb, according to the manufacturer's instructions. Then, one of the mAbs that was biotinylated was mixed in a test tube with several dilutions of 1 of the 2 other mAbs which were not biotinylated. Next, the solution was added to the ELISA plate coated with nCan f 4 (1 µg/mL). After an incubation of 1 hour at 37℃, the bound biotinylated mAb was detected with Streptavidin-HRP (1:10,000, GE Healthcare). All 6 combinations were tested. The color reaction was developed by the TMB Single Solution reagent (Zymed Laboratories, Inc. San Francisco, CA, USA) and measured at 450 nm. The results were expressed as inhibition percentages.
Experimental dust samples were collected from homes with and without dogs (5 and 4 samples, respectively) by vacuum cleaning the carpet in the living room for 5 minutes. They were extracted overnight in PBS (1:10 w/v, pH 7.4) at 4℃. After centrifugation and sterile filtration, the protein concentration of the extracted samples was determined by the Bio-Rad Protein Assay Kit using bovine serum albumin as a standard.
For measuring the Can f 4 content in the samples, microtiter plates were coated with mAb 26D (5 µg/mL) overnight at 4℃. Then test samples (diluted in PBS containing 1% BSA/0.05% Tween 20), and rCan f 4 standards (0.01 to 2,500 ng/mL) were incubated at 37℃ for 2 hours. After an incubation (1 hour, 37℃) with biotinylated mAb 48F mAb (1 µg/mL), followed by an incubation with Streptavidin-HRP (1:10,000, GE Healthcare, Buckinghamshire, UK, 0.5 hour, room temperature), the color reaction was developed and measured, as described above. To determine the amount of Can f 4 in test samples, a standard curve of absorbance against the log concentration of the rCan f 4 standard was plotted. The results were calculated from the straight part of this curve.
Levels of IgE antibodies to the Can f 4 preparations were measured with indirect ELISAs, as previously described,7 with a few modifications. The Nunc® MaxiSorp microtiter plates (Thermo Fisher Scientific, Roskilde, Denmark) were first coated with nCan f 4, rCan f 4 or multimeric form (mu) of rCan f 4 (5 µg/mL) and then incubated with sera diluted 1:10 for 1 hour at 37℃. The diluent contained 2% of mouse serum (Sigma-Aldrich). The bound IgE was detected by biotinylated mouse anti-human IgE (1:1,000; Southern Biotechnology Associates, Inc., Birmingham, AL, USA) combined with Streptavidin-HRP (1:10,000). The color reaction was developed by the TMB Single Solution reagent. The results were expressed as optical density (OD) values at 450 nm. The cutoff for a positive reaction was defined as the mean OD of the healthy control subjects plus 3 SD.
Patients' IgE reactivity to Can f 4 in the commercial SPT dog dander extract (ALK Abelló) and to the purified allergens nCan f 4 and rCan f 4 was detected with SDS-PAGE immunoblotting, basically as previously reported.21,22 The same technique was used in detecting the reactivity of the mAbs 26D, 41G, and 48F against the commercial extract and the in-house dog dander extract. In brief, the protocol used for all Western blotting experiments was as follows: After SDS-PAGE and protein transfer, the membrane strips with the dog dander extract or the allergens were incubated overnight with sera (diluted 1:10) or each of the mAbs (0.5 µg/mL), followed by incubation for 2 hours with monoclonal horseradish peroxidase-labeled mouse anti-human IgE at 1:2,000 or ZyMax™ rabbit-anti-mouse IgG-HRP (Zymed Laboratories) at 1:5,000, respectively. The reactive bands were visualized with the ECL Western Blotting Detection Reagents (GE Healthcare).
The reactivity of antibodies to the denatured or nondenatured nCan f 4 was analyzed by the dot blot method. The quantification of dot intensities was performed by using the public domain ImageJ software (http://imagej.nih.gov/ij/)23 and the MicroArray Profile.jar algorithm (http://www.optinav.com/download/MicroArray_Profile.jar). For details, see Supplementary Material 1.
Individual serum samples or pooled sera from 6 dog-allergic patients with diverse levels of specific IgE to Can f 4 or the Can f 4-specific mAbs produced were used in ELISA inhibitions, basically as previously described.14 In brief, the tests were performed essentially as the indirect ELISAs but with an additional preincubation (1 hour at 37℃) of the sera or the mAbs with the inhibitor proteins (nCan f 4, rCanf 4 , rCan f 1, rCan f 2, rEqu c 1, rBos d 2, rMus m 1, rTL, or rPsoriasin). In the preincubation phase, the final serum dilution ranged from 1:30 to 1:75 depending on the IgE level. In ELISA inhibitions with the biotinylated mAbs, appropriate dilutions were used. The final concentrations of the inhibitors ranged from 0.3 to 200 µg/mL. The results were expressed as inhibition percentages.
All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and SPSS (IBM SPSS Statistics 21 I; IBM Corp, Armonk, NY, USA) The two-tailed t test and the Mann-Whitney U test were used for determining statistically significant differences between the groups. For assessing the inter-rater agreement (categorical items) and for analyzing correlations, the Kappa (κ) statistic and the Spearman Rank correlation test were used, respectively.
To generate mAbs specific to Can f 4, a protein fraction with a molecular mass of 12-18 kDa7 was isolated by size exclusion chromatography (SEC) from in-house dog dander extract and used for immunizing mice. Exploiting the 3 mAbs (41G, 26D, and 48F) with reactivity to the 18kDa component in dog dander (Fig. 1), nCan f 4 was isolated from the extract by affinity chromatography. Its high level of purity was verified by silver-stained SDS-PAGE (Fig. E1A in Supplementary Material 2). The glycoprotein staining of the nCan f 4 protein suggested that the protein is nonglycosylated (data not shown).
The cDNA encoding the Can f 4 (GenBank accession number KF192077) was amplified from a mixture of cDNAs derived from dog parotid gland, mandibular gland, and tongue tissues. It was found that the cDNA contains an open reading frame encoding a 174 amino-acid protein. At the beginning, there is a putative 16 amino-acid leader, followed by a 158 amino-acid mature protein. The predicted molecular mass is 17.6 kDa, and the predicted pI is 6.19. Comparison of the amino acid sequence with the previously published one8 revealed 4 differences: Ile36Val, Asp46Glu, Met54Leu, and Ser66Leu (Fig. E2 in Supplementary Material 2). In order to investigate the importance of Can f 4 as an allergen, it was produced as a recombinant protein using the P. pastoris expression system.
We have previously reported that 60% of dog dust-sensitized subjects exhibited IgE reactivity to a band of dog dander extract corresponding to Can f 4 in immunoblotting.7 In contrast, Mattsson et al.8 found that only 35% of dog-allergic subjects were sensitized to rCan f 4 as examined by ImmunoCAP (Phadia, Uppsala, Sweden). Here, we first examined the IgE reactivity of 63 dog-allergic patients and 12 control subjects to the isolated nCan f 4 by immunoblotting. Twenty-nine (46%) out of the 63 allergic subjects showed IgE binding to the natural allergen (Fig. E4 in Supplementary Material 2), thus pointing to a possibility that Can f 4 is recognized less frequently by dog-allergic subjects than previously assumed. In the immunoblotting analysis with rCan f 4, 31 (49%) out of the 63 subjects showed IgE reactivity to the allergen. The agreement between the 2 analyses was very good, as 29 samples were positive with both allergen preparations (κ=0.94, P<0.001). None of the control subjects was positive in the analyses (Fig. E4 in Supplementary Material 2).
IgE reactivity to the Can f 4 preparations was further evaluated by indirect ELISAs. In comparison to the immunoblot results, a greatly increased prevalence of IgE reactivity to the immunoaffinity-purified nCan f 4 was found: 51 out of the 63 serum samples (81%) were positive (Fig. 2A). However, when the sera were analyzed for specific IgE to rCan f 4, the prevalence of IgE reactivity decreased to 54%, as only 34 out of the 63 sera were positive (Fig. 2A). Therefore, the agreement between the analyses was only fair (κ=0.37, P<0.001). Interestingly, when multimeric (mu) rCan f 4 was used as antigen at the same protein concentration, 39 samples (62%) were found to be positive (Fig. 2A), and the agreement with the nCan f 4-based ELISA was better (κ=0.40, P<0.001). The specific IgE levels of dog-allergic subjects to nCan f 4, rCan f 4, or murCan f 4 were comparable (Fig. 2A; P>0.05, two-tailed t test). The prevalences of Can f 4-specific IgE reactivities among Finnish and Spanish dog-allergic patients according to the assays and allergen preparations used are shown in Fig. 2B.
The results above hint that the discrepancy in IgE seropositivity to Can f 4 with different methods and that allergen preparations could be due to the conformation dependency of IgE binding. To examine this, a dot blot analysis with the 3 mAbs and 3 dog-allergic patients' sera was conducted using natural undenatured or denatured Can f 4. Fig. E5 shows (see Supplementary Material 2) that denaturation greatly attenuate antibody binding to the natural allergen. On average, the intensity of dot blot reactions reduced by 44.8% with mAbs and by 54.4% with sera analyzed (analysis by ImageJ Microarray).
Sixteen dog-allergic patients and 6 nonatopic volunteers were examined with SPTs for reactivity to the natural and recombinant Can f 4 preparations. Fourteen (88%) and 7 (44%) out of the 16 allergic subjects showed a positive reaction to nCan f 4 or rCan f 4, respectively. Therefore, the agreement between the tests was only fair (κ=0.20, P=0.18). All the control subjects were negative in the tests. The mean wheal diameters induced by nCan f 4 (4.4±0.51 [mean±SEM] mm) and rCan f 4 (3.8±0.92 [mean±SEM] mm) did not differ statistically (P>0.05, Mann Whitney U test), and there was a good correlation between the wheal sizes (Spearman rank correlation; r=0.89, P<0.001).
To quantitatively compare the antibody-binding capacities of recombinant and natural Can f 4, the reactivity of each mAb to rCan f 4 was inhibited with nCan f 4 in ELISA, and then vice versa. As shown in Fig. 3A, the binding of mAbs to rCan f 4 was completely inhibited with nCan f 4. The reverse experimental setting (inhibition with rCan f 4) produced the same result (Fig. 3A). However, the inhibition curve with mAb 41G did not overlap entirely between the preparations (as was the case with 26D and 48F), as nCan f 4 was needed about 10 times more than the recombinant preparation to obtain the 50% inhibition level (Fig. 3A).
IgE-binding capacities of the Can f 4 preparations were further examined by IgE ELISA inhibitions. As shown in Fig. 3B, the IgE reactivity of 3 individual sera to rCan f 4 was completely inhibited by prior incubation with nCan f 4, whereas the reverse was not; rCan f 4 inhibited 50%-70% of the reactivity to nCan f 4.
The specificity of IgE binding to Can f 4 was examined by ELISA inhibition using nCan f 4 in the solid phase. As shown in Fig. 4, none of the 6 lipocalin proteins or the control proteins psoriasin or tear lipocalin showed IgE cross-reactivity with Can f 4.
For measuring the amount of Can f 4 in environmental samples or allergen preparations, a sandwich ELISA was developed. To that end, the 3 Can f 4-specific mAbs (all IgG1, data not shown) were analyzed by competitive ELISAs to provide an optimal pair for capturing Can f 4. Fig. 5A demonstrates that 2 of the antibodies (41G and 48F) recognize the identical or overlapping epitope. Therefore, after further experiments, the mAbs 26D and 48F were adopted for the ELISA. Fig. 5B shows that the linear part of the ELISA curve lays between the Can f 4 concentrations of 10 and 1,000 ng/mL. Next, we measured the concentrations of Can f 4 in the experimental dust samples collected with a vacuum cleaner from 5 homes with and 4 homes without dogs. Fig. 5C shows that the variation of the measured Can f 4 concentration is large. The mean Can f 4 concentration in the samples from homes with dogs was 67.3±61.0 µg/g of dust whereas it was 4.0±0.1 ng/g in the samples from homes without dogs.
To clarify the significance of Can f 4 in dog allergy, we both purified the natural allergen with mAb-based affinity chromatography from dog dander extract and cloned its new variant in a recombinant form in Pichia pastoris. These 2 products were used to determine the prevalence of IgE reactivity to the allergen among dog-allergic subjects by ELISA, SPTs, and Western blotting.
Our results show that Can f 4 is one of the major allergens from the dog. However, we found that the IgE-binding capacity of the immunoaffinity-purified nCan f 4 is very sensitive to denaturation. For example, when we assessed the prevalence of IgE reactivity to the allergen by ELISA (antigen not denatured) and Western blotting (antigen only partly renatured24), the IgE prevalence was found to be strongly reduced by the latter method. This phenomenon was equally seen with both Finnish and Spanish dog-allergic patients' sera (Fig. 2B). Mostly, the missing IgE reactivity in immunoblotting involved the sera which showed low IgE levels in ELISA, up to OD 0.3, but there were also a few sera with higher IgE levels that lost their IgE reactivity in immunoblotting (data not shown). Further tests with the dot blot technique, which allows an accurate way to quantify the intensity of IgE reaction, showed that the denaturation of nCan f 4 diminishes its antibody-binding capacity strongly compared to the undenatured preparation. Therefore, the impairment of conformation of nCan f 4 may explain the lower prevalence of IgE reactivity to it in Western blotting in which proteins are known to be incompletely renatured.24,25,26,27
We cannot tell as yet whether the pronounced sensitivity of nCan f 4 to denaturation and the resulting loss of IgE binding are features specific for nCan f 4 among lipocalin allergens. We have previously found, however, a similar phenomenon with Bos d 2, a major cow dander allergen, in that about 60% of cow dust-sensitized subjects showed IgE reactivity in SDS-PAGE immunoblotting to the natural allergen,28 whereas more than 80% of subjects with a similar disease had IgE to rBos d 2 in ELISA.14 As it is well known that disturbing the 3-dimensional structure of allergens, including lipocalins, results in diminished IgE binding,29,30,31 it seems plausible that Western blotting, because of denaturation, underestimates the frequency of IgE reactivity to nCan f 4. As Hattori et al.32 reported the complete refolding of β-lactoglobulin, a lipocalin, was found to require disulfide bond formation under strict conditions. Our findings are compatible with this view, as our analysis showed that immunoaffinity-purified nCan f 4 contains a functional disulfide bridge (Fig. E1B in Supplementary Material 2). Indeed, several studies have found that the structural integrity of allergens is crucial for maintaining conformational epitopes and recognition by IgE.33,34,35
We infer that the observed difference in the prevalence of IgE reactivity by nCan f 4 and rCan f 4-specific indirect ELISAs (Fig. 2A) also reflects the propensity of Can f 4 to lose its 3 dimensional structure, as rCan f 4 was apparently missing some of the epitopes which the immunoaffinity-purified nCan f 4 contains. Accordingly, the prevalence of positive SPT results with rCan f 4 was lower than that with nCan f 4. As the prevalences of nCan f 4 and rCan f 4 IgE determined by Western blotting were in good agreement and the IgE reactivity to rCan f 4 was not significantly enhanced in nondenaturing conditions (ELISA and SPTs), it is conceivable that the eventual shape of rCan f 4 does not match quite perfectly to that of nCan f 4.
Many allergens have been found to have a tendency to multimerize in their crystal structures and to form transient dimers in solution.18,36 Oligomerization is also a mechanism for the generation of additional conformational IgE epitopes on allergenic proteins37,38 For rCan f 4, we revealed, by size exclusion chromatography, that the allergen preparation contained oligomeric forms (Fig. E3 in Supplementary Material 2). It is important to note, however, that only monomers were visible in the SDS-PAGE analysis of the multimeric rCan f 4, indicating that the multimers decompose when they are denatured (data not shown). To test the hypothesis that the multimeric form of the allergen could have a higher IgE binding capacity than the monomeric one, we measured the IgE reactivity of dog-allergic subjects to murCan f 4 in ELISA and found, indeed, a detectable rise in IgE prevalence. Therefore, it is possible that the natural presentation of Can f 4 in dog dander also includes oligomers, a property shared with some other lipocalins.36,39,40 To some extent, this property can also be a factor underlying the sensitivity of Can f 4 to denaturation.
It is well known that allergens, including mammal-derived respiratory allergens of the lipocalin family, such as Can f 4, can have variants that differ from each other by a few amino acids.41,42 The sequence we obtained for Can f 4 differs by 4 amino acids from that reported by Mattsson et al.8 ELISA inhibition analyses corroborated the view that rCan f 4 and immunoaffinity-purified nCan f 4 are not completely identical because the ELISA inhibition curves did not overlap completely with 2 of the mAbs, even though both allergen preparations could entirely inhibit the binding of all mAbs to the counterpart allergen at high protein concentrations (Fig. 3A). Furthermore, IgE ELISA inhibitions proved that rCan f 4 is devoid of some of the epitopes the nCan f 4 preparation contains because rCan f 4 could not inhibit all IgE binding to nCan f 4 (Fig. 3B). Our studies do not rule out a possibility that the immunoaffinity-purified nCan f 4 preparation would comprise more than 1 Can f 4 variant or isoallergen. As the commercial dog dander raw material we used as a source of nCan f 4 was a mixture from different dog breeds, it is not possible to know whether these supposed allergen variants or isoforms are related to individual dogs or breeds.
There are several reports describing IgE cross-reactivity between mammalian lipocalin allergens.9,14,43,45 Mostly, the cross-reactivity appears to reflect the sequential similarity of the proteins. However, a similar 3 dimensional structure with low sequential similarity appears to be sufficient in some cases.14,45 In this study, we did not observe any IgE cross-reactivity between Can f 4 and the lipocalin allergens tested (Fig. 4). Interestingly, Mattson et al.8 showed, in their study, that Can f 4-reactive sera of dog allergic subjects detect a 23-kDa protein in a cow dander extract.
We also describe here 3 mAbs which we used successfully in purifying and analyzing the dog allergen Can f 4. We carefully selected the mAbs 26D and 48F for their epitope specificities to develop a sandwich ELISA for measuring the allergen. The feasibility of the new immunoassay was assessed by determining Can f 4 content in experimental house dust samples collected from 9 homes (5 homes with and 4 homes without dogs). The level of Can f 4 in dust samples from households with dogs was comparable to that of Can f 1 in dust samples from environments in which dogs are present.46,47
In conclusion, this study ensures the relevance of Can f 4 in dog allergy. Our results show that this allergen is one of the major allergens from the dog together with Can f 16 and Can f 5.11 However, the sensitivity of the allergen for denaturation underlines that different methods and allergen preparations can produce divergent results on the prevalence of allergic sensitization.
Figures and Tables
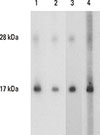 | Fig. 1Monoclonal antibodies and a dog-allergic patient's serum IgE detect the Can f 4 allergen in the dog dander extract (ALK Abelló). Lane 1, mAb 41G; lane 2, mAb 26D; lane 3, mAb 48F; lane 4, patient serum (tested earlier to be Can f 1- and Can f 2-negative). |
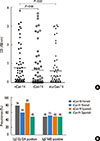 | Fig. 2IgE reactivity to the Can f 4. (A) Serum IgE reactivity of 63 dog-allergic patients to nCan f 4, rCan f 4 and murCan f 4 in ELISA. The prevalence of IgE reactivity to the preparations was 81%, 54% and 62%, respectively. There were no statistically significant differences in the IgE level between the allergen preparations (P>0.05, Two-tailed t-test). Horizontal lines represent the mean values and the dotted lines the cut-off values (mean OD value of healthy control subjects plus 3 SD) for each ELISA test. OD, optical density. (B) IgE prevalence to nCan f 4 and rCan f 4 among Finnish (gray and blue, respectively) and Spanish (orange and green bars, respectively) dog-allergic patients assayed with ELISA and Western blotting. The cut-off for a positive ELISA reaction was defined as the mean OD of the healthy control subjects plus 3 SD.n, natural; r, recombinant; mu, multimeric.
|
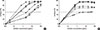 | Fig. 3ELISA inhibition analyses. Varying concentrations of natural Can f 4 (open symbols) or recombinant Can f 4 (closed symbols) were used to inhibit the binding of mAbs A or serum IgE B to the plate-bound counterpart allergen. (A) mAbs 26D (circles), 41G (triangles) and 48F (tetragons) were preincubated with the inhibitors and the residual antibody binding to the counterpart allergen was measured. (B) Three individual patient serum samples (circles, triangles, and tetragons) were preincubated with the inhibitors and the residual IgE binding to the counterpart allergen was measured. Data are expressed as percentages relative to the binding of an antibody without an inhibitor. |
 | Fig. 4ELISA inhibition of IgE binding to the natural Can f 4 allergen by lipocalin proteins. A pooled serum sample from six dog-allergic patients with IgE reactivity to nCan f 4 was preincubated with recombinant lipocalin allergens (rCan f 1, rCan f 2, rEqu c 1, rMus m 1, rBos d 2), or nCan f 4 as a positive inhibition control, or rTL and rPsoriasin as negative controls, and the residual IgE binding to the allergen was measured. n, natural; r, recombinant. |
 | Fig. 5Can f 4-specific mAbs and the sandwich ELISA for the quantification of Can f 4. (A) The epitope specificity of the three Can f 4-specific mAbs analyzed by competitive ELISA. Binding of the biotinylated mAbs to nCan f 4 was inhibited with the same mAbs which were not biotinylated. Percentages of inhibition are indicated. (B) The linear part of the recombinant Can f 4 standard curve is between 10 and 1,000 ng/mL in the sandwich ELISA. (C) Can f 4 concentrations of experimental home dust samples measured by the mAb-based sandwich ELISA. Horizontal lines represent the mean values. |
ACKNOWLEDGMENTS
We thank Maria Basagana, from the Allergy Department, Institut Universitari Dexeus, Universitat Autonoma de Barcelona, Spain, for providing a set of serum samples from dog-allergic subjects. We also thank Mirja Saarelainen and Merja Esselström for their skillful technical assistance.
This work was financially supported by the Kuopio university Hospital, project 5021605.
References
1. Perzanowski MS, Rönmark E, Nold B, Lundbäck B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999; 103:1018–1024.
2. Partti-Pellinen K, Marttila O, Mäkinen-Kiljunen S, Haahtela T. Occurrence of dog, cat, and mite allergens in public transport vehicles. Allergy. 2000; 55:65–68.
3. Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008; 38:1635–1643.
4. Munir AK, Einarsson R, Schou C, Dreborg SK. Allergens in school dust. I. The amount of the major cat (Fel d I) and dog (Can f I) allergens in dust from Swedish schools is high enough to probably cause perennial symptoms in most children with asthma who are sensitized to cat and dog. J Allergy Clin Immunol. 1993; 91:1067–1074.
5. World Health Organization. International Union of Immunological Societies, Allergen Nomenclature- Sub-committee. Allergen nomenclature [Internet]. [place unknown]: Allergen Nomenclature Sub-committee;cited 2014 Dec 12. Available from: http://www.allergen.org.
6. Konieczny A, Morgenstern JP, Bizinkauskas CB, Lilley CH, Brauer AW, Bond JF, et al. The major dog allergens, Can f 1 and Can f 2, are salivary lipocalin proteins: cloning and immunological characterization of the recombinant forms. Immunology. 1997; 92:577–586.
7. Saarelainen S, Taivainen A, Rytkönen-Nissine M, Auriola S, Immonen A, Mäntyjärvi R, et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy. 2004; 34:1576–1582.
8. Mattsson L, Lundgren T, Olsson P, Sundberg M, Lidholm J. Molecular and immunological characterization of Can f 4: a dog dander allergen cross-reactive with a 23 kDa odorant-binding protein in cow dander. Clin Exp Allergy. 2010; 40:1276–1287.
9. Hilger C, Swiontek K, Arumugam K, Lehners C, Hentges F. Identification of a new major dog allergen highly cross-reactive with Fel d 4 in a population of cat- and dog-sensitized patients. J Allergy Clin Immunol. 2012; 129:1149–1151.
10. Pandjaitan B, Swoboda I, Brandejsky-Pichler F, Rumpold H, Valenta R, Spitzauer S. Escherichia coli expression and purification of recombinant dog albumin, a cross-reactive animal allergen. J Allergy Clin Immunol. 2000; 105:279–285.
11. Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol. 2009; 123:362–368.
12. Ylönen J, Mäntyjärvi R, Taivainen A, Virtanen T. IgG and IgE antibody responses to cow dander and urine in farmers with cow-induced asthma. Clin Exp Allergy. 1992; 22:83–90.
13. van Ree R, van Leeuwen WA, Bulder I, Bond J, Aalberse RC. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol. 1999; 104:1223–1230.
14. Saarelainen S, Rytkönen-Nissinen M, Rouvinen J, Taivainen A, Auriola S, Kauppinen A, et al. Animal-derived lipocalin allergens exhibit immunoglobulin E cross-reactivity. Clin Exp Allergy. 2008; 38:374–381.
15. Kauppinen A, Peräsaari J, Taivainen A, Kinnunen T, Saarelainen S, Rytkönen-Nissinen M, et al. Association of HLA class II alleles with sensitization to cow dander Bos d 2, an important occupational allergen. Immunobiology. 2012; 217:8–12.
16. Zeiler T, Mäntyjärvi R, Rautiainen J, Rytkönen-Nissinen M, Vilja P, Taivainen A, et al. T cell epitopes of a lipocalin allergen colocalize with the conserved regions of the molecule. J Immunol. 1999; 162:1415–1422.
17. O'Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997; 18:349–359.
18. Niemi MH, Rytkönen-Nissinen M, Jänis J, Virtanen T, Rouvinen J. Structural aspects of dog allergies: the crystal structure of a dog dander allergen Can f 4. Mol Immunol. 2014; 61:7–15.
19. Rautiainen J, Auriola S, Rouvinen J, Kauppinen J, Zeiler T, Novikov D, et al. Molecular and crystal properties of Bos d 2, an allergenic protein of the lipocalin family. Biochem Biophys Res Commun. 1998; 247:746–750.
20. Porre S, Heinonen S, Mäntyjärvi R, Rytkönen-Nissinen M, Perola O, Rautiainen J, et al. Psoriasin, a calcium-binding protein with chemotactic properties is present in the third trimester amniotic fluid. Mol Hum Reprod. 2005; 11:87–92.
21. Zeiler T, Taivainen A, Rytkönen M, Rautiainen J, Karjalainen H, Mäntyjärvi R, et al. Recombinant allergen fragments as candidate preparations for allergen immunotherapy. J Allergy Clin Immunol. 1997; 100:721–727.
22. Rautiainen J, Rytkönen M, Virtanen T, Pentikäinen J, Zeiler T, Mäntyjärvi R. BDA20, a major bovine dander allergen characterized at the sequence level, is Bos d 2. J Allergy Clin Immunol. 1997; 100:251–252.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–675.
24. Zampieri S, Ghirardello A, Doria A, Tonello M, Bendo R, Rossini K, et al. The use of Tween 20 in immunoblotting assays for the detection of autoantibodies in connective tissue diseases. J Immunol Methods. 2000; 239:1–11.
25. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979; 76:4350–4354.
26. Zeng FY, Oka JA, Weigel PH. Renaturation and ligand blotting of the major subunit of the rat asialoglycoprotein receptor after denaturing polyacrylamide gel electrophoresis. Glycobiology. 1996; 6:247–255.
27. Aalberse JA, Meijer Y, Derksen N, van der Palen-Merkus T, Knol E, Aalberse RC. Moving from peanut extract to peanut components: towards validation of component-resolved IgE tests. Allergy. 2013; 68:748–756.
28. Ylönen J, Mäntyjärvi R, Taivainen A, Virtanen T. Comparison of the antigenic and allergenic properties of three types of bovine epithelial material. Int Arch Allergy Immunol. 1992; 99:112–117.
29. Ball T, Linhart B, Sonneck K, Blatt K, Herrmann H, Valent P, et al. Reducing allergenicity by altering allergen fold: a mosaic protein of Phl p 1 for allergy vaccination. Allergy. 2009; 64:569–580.
30. Moldaver D, Larché M. Immunotherapy with peptides. Allergy. 2011; 66:784–791.
31. Kauppinen J, Zeiler T, Rautiainen J, Rytkönen-Nissinen M, Taivainen A, Mäntyjärvi R, et al. Mutant derivatives of the main respiratory allergen of cow are less allergenic than the intact molecule. Clin Exp Allergy. 1999; 29:989–996.
32. Hattori M, Hiramatsu K, Kurata T, Nishiura M, Takahashi K, Ametani A, et al. Complete refolding of bovine beta-lactoglobulin requires disulfide bond formation under strict conditions. Biochim Biophys Acta. 2005; 1752:154–165.
33. Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, et al. Engineering of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Nat Biotechnol. 1997; 15:754–758.
34. Schramm G, Bufe A, Petersen A, Haas H, Merget R, Schlaak M, et al. Discontinuous IgE-binding epitopes contain multiple continuous epitope regions: results of an epitope mapping on recombinant Hol l 5, a major allergen from velvet grass pollen. Clin Exp Allergy. 2001; 31:331–341.
35. Varshney S, Goldblum RM, Kearney C, Watanabe M, Midoro-Horiuti T. Major mountain cedar allergen, Jun a 1, contains conformational as well as linear IgE epitopes. Mol Immunol. 2007; 44:2781–2785.
36. Rouvinen J, Jänis J, Laukkanen ML, Jylhä S, Niemi M, Päivinen T, et al. Transient dimers of allergens. PLoS One. 2010; 5:e9037.
37. Schöll I, Kalkura N, Shedziankova Y, Bergmann A, Verdino P, Knittelfelder R, et al. Dimerization of the major birch pollen allergen Bet v 1 is important for its in vivo IgE-cross-linking potential in mice. J Immunol. 2005; 175:6645–6650.
38. Reese G, Ballmer-Weber BK, Wangorsch A, Randow S, Vieths S. Allergenicity and antigenicity of wild-type and mutant, monomeric, and dimeric carrot major allergen Dau c 1: destruction of conformation, not oligomerization, is the roadmap to save allergen vaccines. J Allergy Clin Immunol. 2007; 119:944–951.
39. Japaridze T, Senda A, Nozaki H, Yanagida M, Suzuki T, Ganzorig K, et al. Cloning, monoclonal antibody production, and bodily distribution pattern of a bovine lipocalin. Biosci Biotechnol Biochem. 2012; 76:712–720.
40. Gu Y, Liu Q, Chen P, Guo C, Liu Y, Zhao Y, et al. Characterization of the oligomerization and ligand-binding properties of recombinant rat lipocalin 11. Biochim Biophys Acta. 2013; 1834:1–7.
41. Rautiainen J, Auriola S, Konttinen A, Virtanen T, Rytkönen-Nissinen M, Zeiler T, et al. Two new variants of the lipocalin allergen Bos d 2. J Chromatogr B Biomed Sci Appl. 2001; 763:91–98.
42. Virtanen T, Kinnunen T, Rytkönen-Nissinen M. Mammalian lipocalin allergens--insights into their enigmatic allergenicity. Clin Exp Allergy. 2012; 42:494–504.
43. Smith W, Butler AJ, Hazell LA, Chapman MD, Pomés A, Nickels DG, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004; 34:1732–1738.
44. Nilsson OB, Binnmyr J, Zoltowska A, Saarne T, van Hage M, Grönlund H. Characterization of the dog lipocalin allergen Can f 6: the role in cross-reactivity with cat and horse. Allergy. 2012; 67:751–757.
45. Madhurantakam C, Nilsson OB, Uchtenhagen H, Konradsen J, Saarne T, Högbom E, et al. Crystal structure of the dog lipocalin allergen Can f 2: implications for cross-reactivity to the cat allergen Fel d 4. J Mol Biol. 2010; 401:68–83.
46. Mussalo-Rauhamaa H, Reijula K, Malmberg M, Mäkinen-Kiljunen S, Lapinlampi T. Dog allergen in indoor air and dust during dog shows. Allergy. 2001; 56:878–882.
47. Vredegoor DW, Willemse T, Chapman MD, Heederik DJ, Krop EJ. Can f 1 levels in hair and homes of different dog breeds: lack of evidence to describe any dog breed as hypoallergenic. J Allergy Clin Immunol. 2012; 130:904–909.e7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download