Abstract
Purpose
Chronic rhinosinusitis with nasal polyps (CRSwNP), a mainly Th2 cytokine-mediated disease, often involves mucus secretion. Recent evidence suggests that transmembrane protein 16A (TMEM16A), a calcium-activated Cl- channel (CaCC), can regulate mucus secretion from airway epithelium by transepithelial electrolyte transport and hydration. However, the role of TMEM16A in mucin production/secretion in the airway epithelium is not clear. This study was conducted to determine the role of TMEM16A in mediating mucin secretion in human nasal polyp epithelial cells (HNPECs) induced by IL-13.
Methods
Human sinonasal mucosa tissue and dissociated sinonasal epithelium from control subjects and patients with CRSwNP were assessed for the expression of TMEM16A and the secretion of human mucin 5AC (MUC5AC) by immunohistochemistry, Western blot analysis, and enzyme-linked immuno-sorbent assay (ELISA). A model of the Th2 inflammatory environment was created by exposure of primary air-liquid interface (ALI)-cultured HNPECs to interleukin-13 (IL-13) for 14 days, with subsequent assessment of TMEM16A expression in cell lysates by Western blotting and MUC5AC secretion in apical washings of cells by ELISA.
Results
The expressions of TMEM16A and MUC5AC were increased in human nasal polyp tissue and dissociated nasal polyp epithelium. TMEM16A was detected in IL-13-treated HNPECs, specifically in MUC5AC-positive cells but not in ciliated cells. IL-13 treatment increased percentages of TMEM16A-positive cells, MUC5AC-positive cells, and cells coexpressing TMEM16A/MUC5AC, the expression of TMEM16A protein, and the secretion of MUC5AC. T16Ainh-A01, a TMEM16A inhibitor, attenuated these IL-13-induced effects.
Conclusions
The expression of TMEM16A and MUC5AC are increased in CRSwNP, which might be a direct effect of Th2 cytokines present in the sinonasal mucosa in CRSwNP. Down-regulation of TMEM16A expression and MUC5AC secretion in HNPECs by T16Ainh-A01 indicates that TMEM16A might play an important role in mucin secretion in upper airway inflammatory diseases.
Patients with nasal polyps often suffer from progressive nasal congestion, hyposmia, and rhinorrhea, which are frequently confused with symptoms of allergic rhinitis and chronic sinusitis.1,2,3 This is especially the case in a patient with chronic rhinosinusitis with nasal polyps (CRSwNP), who suffers from prolonged rhinorrhea even after adequate antimicrobial treatment.
CRSwNP is a disorder characterized by persistent eosinophilic Th2 inflammation and frequent sinonasal microbial colonization.4 Studies of cytokines associated with chronic rhinosinusitis have showed that CRSwNP or CRS without nasal polyps (CRSsNP) have a mixed Th1/Th2 cytokine profile, indicating that cytokines play an important pathologic role in these conditions.4,5,6,7,8,9,10,11 A recent study investigating the expression of various cytokines in nasal epithelial cell cultures of CRSwNP or CRSsNP patients and controls has demonstrated that IL-4 and IL-13 are increased in CRSwNP, whereas IL-4 is increased in CRSsNP, compared to controls.5 Ramanathan et al.4 created a model of the Th2 inflammatory environment based on exposure of primary human sinonasal epithelial cells (HSNECs) to the Th2 cytokines interleukin IL-4 or IL-13 for 36 hours and demonstrated that treatment of HSNECs with IL-4 or IL-13 significantly down-regulates the baseline expression of antimicrobial innate immune genes and leads to microbial colonization and abnormal immune responses associated with CRSwNP. Another recent study compared the levels of eosinophil chemoattractant cytokines in nasal lavage fluid and nasal polyp tissue extracts from CRSwNP patients and showed that IL-5 and IL-13 and RANTES are elevated in parallel in nasal lavage fluid and nasal polyps from these patients.9 Furthermore, IL-13 is also elevated in nasal polyp compared to control uncinate processes (UP) and CRSsNP UP.9 Similarly, the serum levels of IL-13 have also been shown to be significantly higher in patients with CRSwNP than in healthy controls,10 and coincide with significantly higher levels of total IgE in patients with CRSwNP, possibly predisposing them to concomitant asthma.10,11
Increasing evidence suggests that calcium-activated chloride channels (CaCCs) play an important role in transepithelial transport and mucus overproduction in the airway epithelium.12,13 Although the molecular identity of CaCCs is not entirely clear, several membrane proteins, such as calcium-activated chloride channel protein (CLCA),14 Bestrophins,15,16 and Tweety,17 have been postulated as candidates. However, electrophysiological characterization of these candidate channels has indicated that Cl- currents and Ca2+-mediated current-voltage relationships for these channels are actually different from those of classical CaCCs.18 More recently, using different approaches, 3 laboratories have simultaneously reported that the molecule transmembrane protein 16A (TMEM16A) is a strong candidate for CaCCs.19,20,21 These studies demonstrated that the expression of TMEM16A in different cell systems (HEK-293 and FRT cells, and Axolotl oocytes) consistently leads to the appearance of voltage-dependent Ca2+-activated Cl- channel, namely, classical CaCCs.19,20,21 Indeed, while Yang et al.20 have shown TMEM16A to be a bona fide Ca2+-activated chloride channel that is activated by intracellular Ca2+ and Ca2+-mobilizing stimuli, Caputo et al.,21 using global gene expression analysis to identify IL-4-regulated CaCC membrane proteins, have shown TMEM16A to be an intrinsic constituent of the calcium-dependent chloride channel. It has been demonstrated that TMEM16A plays an important role in the regulation of airway fluid and electrolyte transport in airway epithelial cells22 and that TMEM16A knockout mice die soon after birth because of significant mucus accumulation in the trachea and tracheomalacia.23
Very few studies have been performed to investigate the role of TMEM16A in mucin secretion. In the lower airways, benzbromarone, as a TMEM16A inhibitor of high-throughput screening, blocks ATP-induced CaCC current and prevents ATP-induced mucin secretion by MUC5AC staining of the mucin stores in normal human bronchial epithelial cells treated with IL-13.24 However, the expression of TMEM16A in nasal polyp tissue and the role of TMEM16A in mucin secretion from nasal polyp epithelial cells induced by IL-13 remain unknown. Meanwhile, the role of T16Ainh-A01 as a TMEM16A inhibitor in airways seems to be a matter of debate. Though T16Ainh-A01 causes only partial inhibition of Cl- secretion triggered by Ca2+-elevating agents in human bronchial and intestinal cells, T16Ainh-A01-sensitive current is increased by 8-fold in IL-4-treated cystic fibrosis human bronchial epithelial cells compared to untreated cells.25 Thus, it is possible that T16Ainh-A01 could have a stronger inhibitory effect on CaCC activity in a Th2 inflammatory environment. The aims of this study were to investigate the expressions of TMEM16A and MUC5AC in nasal polyp tissue and to determine whether TMEM16A could regulate mucin secretion by cultured human nasal polyp epithelial cells (HNPECs) induced by IL-13. This study also aimed to determine if TMEM16A-induced effects in HNPECs could be inhibited by T16Ainh-A01.
Twenty patients with CRSwNP and 10 control subjects were enrolled in the study from the Rhinology ward, Department of Otolaryngology, Head and Neck Surgery, Beijing TongRen Hospital. The diagnosis of CRSwNP was made according to the current European EAACI Position Paper on Rhinosinusitis and Nasal Polyps.26 Comprehensive clinical characterics, including nasal symptoms, examination, nasal endoscopy, and computed tomographic scanning of the sinus were made for diagnostic evaluation of each patient. All CRSwNP patients had at least 1 sinonasal polyp, as determined by nasal endoscopy before or at the time of endoscopic sinus surgery, and any patient who had received systemic pharmacotherapy for sinusitis, such as macrolide antibiotics or intranasal glucocorticoids, for a period of at least 4 weeks prior to surgery was excluded. Ten adult patients who had maxillary sinus cyst, cerebrospinal fluid rhinorrhea without acute rhinosinusitis, CRSsNP, or allergic rhinitis, were enrolled as control subjects. The atopic status of each patient was evaluated by skin prick tests (SPT), using a standard panel of 20 common regional inhalant allergens. Six of the patients with CRSwNP were SPT-positive, and all the control subjects were SPT-negative.
The study protocol was reviewed and approved by the local ethics board of Beijing TongRen Hospital, Capital Medical University, and the study was performed at the Department of Otolaryngology, Head and Neck Surgery, Beijing TongRen Hospital. Written informed consent was obtained from all subjects prior to their participation in the study.
Human nasal polyps from CRSwNP patients and normal sinonasal mucosa tissues from control subjects were obtained during endoscopic sinus surgery, and immediately placed in sterile cold saline. All samples were washed with D-hanks balanced salt solutions supplemented by penicillin (100 U/mL; Gibco, Carlsbad, CA, USA), streptomycin (100 µg/mL; Gibco), amphotericin B (2.5 µg/mL; Gibco) and incubated in 0.5% protease (type XIV; Sigma, St.Louis, MO, USA) in Dulbecco's Modified Eagle's Medium (DMEM; Gibco) overnight at 4℃. Following incubation, the protease was neutralized with an equal volume of DMEM containing 10% bovine serum, and the epithelial cells were released from the tissues by vigorous shaking. The cells were harvested after centrifugation and resuspension in fresh DMEM and plated on a plastic dish at 37℃ for 1 hour to eliminate fibroblasts, endothelial cells, and myoblasts.
The dissociated epithelial cells were seeded into collagen-coated 100-mm tissue culture dishes and cultured in serum-free bronchial epithelial growth medium (BEGM) at 37℃ in an atmosphere of 5% CO2 and 95% relative humidity. The culture medium was changed every 2 days; when the cultures reached 70%-80% confluence, the cells were detached with 0.05% trypsin-EDTA (Gibco). The detached cells were washed with fresh medium and then seeded on 6- or 24-well BD falcon cell culture inserts (23.1 mm or 6.4mm, polyester 0.4 µm, Becton Dickinson Corp [BD], Bedford, MA, USA) at a density of 2×105 cells/cm2. Both sides of the Transwell were filled with BEGM: DMEM (50:50); supplemented with hydrocortisone (0.5 µg/mL), insulin (5 µg/mL), transferrin (10 µg/mL), epinephrine (0.5 µg/mL), triiodothyronine (6.5 µg/mL), gentamycin (50 µg/mL), retinoic acid (0.1 ng/mL), and epidermal growth factor (0.5 ng/mL human recombinant) (all supplied by Clonetics Corp, San Diego, CA, USA); and culture as above. On reaching confluence, the apical medium was removed and cells were cultured further at an air-liquid interface at 37℃ in an atmosphere of 5% CO2 and 95% relative humidity.
Medium containing IL-13 (10 ng/mL; PeproTech, Rocky Hill, NJ, USA) was added to the basolateral side of the inserts from beginning of ALI culture for 14 days as described before.27,28 Likewise, medium containing T16Ainh-A01 (10 µM; Tocris, Bristol, UK), a TMEM16A inhibitor, was added in the same manner. Control wells were cultured with 0.5 mL of ALI medium alone.
Freshly obtained sinonasal mucosal tissues from control subjects and patients with CRSwNP were assessed for the presence of TMEM16 A and MUC5A by immunohistochemistry. Briefly, all samples were fixed with 10% paraformaldehyde (pH 7.4), embedded in paraffin, and cut into 4-µm thick sections. After autoclave pretreatment, the sections were incubated with 3% hydrogen peroxide, rinsed in phosphate buffered solution (PBS), and blocked with 5% skimmed milk, prior to being incubated overnight at 4℃ with TMEM16A antibody (rabbit monoclonal; 1:100; Abcam, Cambridge, MA, USA) or MUC5AC antibody (mouse monoclonal; 1:100; Sigma). The sections were subsequently incubated with peroxidise-conjugated secondary antibody for 40 minutes at 37℃, and at the end of incubation the peroxidase activity was visualized by a colour reaction using diaminobenzidine (DAB) as the substrate. The slides were counterstained with haematoxylin, mounted, and examined using an Olympus BX51 microscope (Tokyo, Japan).
ALI-cultured HNPECs were assessed for the presence of TMEM16 A and MUC5A by immunofluorescence. Briefly, the cultures were fixed in a 50:50 mixture of methanol-acetone, rinsed in PBS, treated with 0.3% triton X-100, and then blocked with 5% skimmed milk prior to being incubated overnight at 4℃ with TMEM16A antibody (rabbit monoclonal; 1:200; Abcam) and MUC5AC antibody (mouse monoclonal; 1:100; Sigma) or acetylated-tubulin antibody (mouse monoclonal; 1:800; Sigma). At the end of this incubation the cells were further incubated with the secondary antibody (fluorescein isothiocyanate-conjugated goat anti-rabbit IgG; 1:100 and rhodamine-conjugated goat anti-mouse IgG; 1:100) for 90 minutes at 37℃, and then counterstained with DAPI nuclear stain. The stained cells were examined using an Olympus IX81 confocal microscope, and image analysis was performed using the Image J software. TMEM16A-positive cells, cells expressing both TMEM16A and MUC5AC, and total number of cells (nuclei) were counted manually inXY fields of 300×300 µm, and the TMEM16A-positive and TMEM16A+MUC5AC-positive cells were expressed as the percentages of total cells.
Sinonasal epithelial or primary cell lysates containing equal amounts of proteins were loaded in separate lanes on a 8% SDS-PAGE gel. After separation, the proteins were transferred onto nitrocellulose membrane, and nonspecific binding sites were blocked by treating with 5% non-fat dry milk powder. The membranes were then treated with primary antibodies directed against TMEM16A (rabbit monoclonal, 1:1,000; Abcam), followed by treatment with secondary HRP-conjugated anti-rabbit (1:20,000) for 1 hour at room temperature. Secondary antibody-bound protein was detected using the Millipore ECL kit. To confirm equal protein loading, blots were reprobed with β-actin antibody (1:5,000). Data were analyzed using the Image J software.
The nasal tissue was processed according to methods as described previously.29 Briefly, snap frozen tissues were weighed, and 1 mL of 0.9% NaCl solution was added per 0.1 g of tissue. The tissue was homogenized with a mechanical homogenizer (Kinematica, Lucerne, Switzerland) at 1,000 rpm for 5 minutes on ice, and the suspension was centrifuged at 3,000 rpm for 10 minutes at 4℃. The supernatants were separated and stored at -80℃ until analysis for MUC5AC using commercial ELISA kits (BlueGene Biotech, Shanghai, China) according to the manufacturer's instruction.
Similarly, ALI-cultured cells were washed 5 times with PBS before being exposed to any stimulation. After different stimulation, apical washings (200 µL of PBS/well, 5 times) were collected and centrifuged at 2,000 rpm for 5 minutes at 4℃. The supernatants were collected and stored at -80℃ until assay for MUC5AC as above.
Statistical analysis was performed using the SPSS17.0 software (SPSS, Inc., Chicago, IL, USA). Data were not available for some assays because of the limited amount of samples obtained at surgery. Data for all assessed samples are expressed as mean±SEM, and any significance of differences between grouped data was calculated using Student's paired t test (two-tailed) or one-way analysis of variance (ANOVA) with post hoc Dunnett's test. A P value of <0.05 was considered significant.
Immunohistochemical staining showed that TMEM16A and MUC5AC proteins were stained strongly in the CRSwNP group (Fig. 1A and B). TMEM16A was expressed throughout the nasal polyp epithelium (the apical side, intracytoplasm, and basolateral side), whereas MUC5A was mostly expressed on the apical side of the nasal polyp epithelium. In contrast, relatively weak expressions of TMEM16A and MUC5AC were noted in normal sinonasal epithelium (Fig. 1C and D). Densitometric analysis of TMEM16A (114 kDa) bands revealed a 4.4-fold increase (Fig. 2A and B), and MUC5AC measured by ELISA was also significantly increased by 6-fold (Fig. 2C) in dissociated human nasal polyp epithelium compared to normal sinonasal epithelium.
To further investigate the localization of TMEM16A and MUC5AC further, we performed immunofluorescence combined with confocal microscopy to assess the expressions of TMEM16A, MUC5AC, and acetylated-tubulin protein (a marker for ciliated cells) in ALI-cultured HNPECs incubated with or without IL-13 (10 ng/mL) for 14 days. TMEM16A expression was mainly expressed in MUC5AC-positive cells in cultured HNPECs incubated with IL-13, with a weak expression noted in the control group. MUC5AC protein was localized in mucus granules, and TMEM16A protein was localized on the circumference of the MUC5AC protein (Fig. 3A and B). TMEM16A and acetylated-tubulin proteins were not coexpressed within the same cells in HNPECs incubated with or without IL-13, indicating that TMEM16A was not expressed on ciliated cells (Fig. 4A and B).
The percentage of TMEM16A-positive cells and MUC5AC-positive cells was significantly higher in HNPECs incubated with IL-13 10 ng/mL for 14 days (23.0%±2.5% and 21.9%±6.0% of total cells, respectively) than in those which was not (4.4%±0.7% and 3.9%±1.5% of total cells, respectively; both P<0.001). Similarly, incubation of cells from control subjects with IL-13 significantly increased the percentage of TMEM16A-positive cells from 3.8%±0.4% to 25.8%±2.1% of total cells (n=6, P<0.001) and MUC5AC-positive cells from 3.5%±1.0% to 24.8%±5.0% (n=6, P<0.001). There was no significant difference in the proportion of TMEM16A-positive and MUC5AC-positive cells between cells of control subjects and the HNPECs group incubated with IL-13 for 14 days (n=6). The proportions of coexpressing TMEM16A and MUC5AC cells incubated with IL-13 in HNPECs (18.6%±1.6%) and epithelial cells from control subjects (21.2%±1.2%) were significantly higher compared than those which were not (n=6, P<0.001) (Fig. 5A). In the 2 groups of cultures incubated with IL-13, the percentage of TMEM16A-positive cells also expressing MUC5AC reached more than 80% of those expressing TMEM16A, respectively.
We further examined the expression of TMEM16A in cell lysates by Western blot analysis and MUC5AC secretion in apical washings of cells by ELISA. Densitometric analysis of TMEM16A bands revealed an approximately 3-fold increase (Fig. 5B and C) and MUC5AC was also increased by approximately 5-fold (Fig. 5D) in the 2 groups incubated with IL-13 compared to the corresponding control groups incubated without IL-13. There was no significant difference in the expressions of the 2 proteins in epithelial cells from control subjects and patients with CRSwNP incubated with IL-13 for 14 days (n=6).
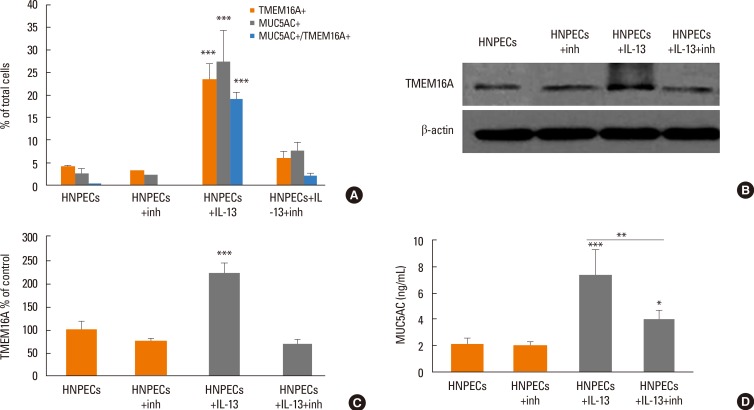
The effect of inhibition of TMEM16A on the expression of TMEM16A and the secretion of MUC5AC induced by IL-13 (10 ng/mL for 14 days) in ALI culture was investigated by incubating the cells with T16Ainh-A01 (10 µM for 14 days). The percentage of TMEM16A-positive cells was significantly decreased from 23.5%±3.4% to 6.1%±1.6% of total cells, and the percentage of MUC5AC-positive cells was decreased from 27.3%±6.9% to 7.6%±2.1% of total cells in HNPECs incubated with IL-13 in the presence of T16Ainh-A01 (n=6, both P<0.001); consequently, the percentage of cell co-expressing TMEM16A and MUC5AC was significantly decreased from 19.1%±1.6% to 2.0%±0.7% of total cells (n=6, P<0.001). T16Ainh-A01 did not significantly decrease the percentage of TMEM16A-positive cells, MUC5AC-positive cells, and cells coexpressing TMEM16A and MUC5AC in HNPECs incubated without IL-13 (Fig. 6A). As shown in (Fig. 6B-D), TMEM16A and MUC5AC protein levels were significantly lower in HNPECs incubated with T16Ainh-A01 plus IL-13 than in those incubated with IL-13 alone. A T16Ainh-A01-induced 68% decrease (P<0.001) in the TMEM16A level was noted in HNPECs incubated with IL-13. Likewise, addition of T16Ainh-A01 significantly decreased MUC5AC secretion by 46.2% (P<0.01) in HNPECs incubated with IL-13.
In this study, we for the first time reported on the relationship between TMEM16A and MUC5AC secretion in CRSwNP in a Th2 inflammatory condition model. Our study demonstrated that the expressions of TMEM16A and MUC5AC were increased in sinonasal mucosa tissue and dissociated sinonasal epithelium from patients with CRSwNP, compared to subjects not suffering from CRS. TMEM16A was detected mainly in MUC5AC-positive cells in HNPECs induced by IL-13. IL-13 treatment also significantly increased the percentage of TMEM16A-positive cells, the percentage of MUC5AC-positive cells, the percentage of cells coexpressing TMEM16A and MUC5AC, the expression of TMEM16A protein, and the secretion of MUC5AC in ALI-cultured sinonasal epithelial cells from control subjects and patients with CRSwNP. Treatment of these cells with T16Ainh-A01, a TMEM16A inhibitor, attenuated the IL-13-induced increases in TMEM16A-positive cells, MUC5AC-positive cells, cells coexpressing TMEM16A and MUC5AC, the expression of TMEM16A, and the secretion of MUC5AC in HNPECs.
A previous study employing an asthmatic mouse model has demonstrated that in airway epithelial cells, TMEM16A is strongly expressed in apical membranes of MUC5AC-positive mucosal cells.24 Another previous study has suggested that TMEM16A is expressed in precursors of goblet cells of bronchial epithelial cells, which may be derived from Clara cells.30 By immunohistochemical analysis, we found that TMEM16A was present not only in the apical cell membrane and on the circumference of MUC5AC protein but also on the basolateral side of nasal polyp epithelium. This finding suggests that in CRSwNP, basal epithelial cells may also easily differentiate into TMEM16A-positive cells. By immunofluorescence staining, our study further indicated that TMEM16A was mainly distributed in most of the MUC5AC-positive cells in HNPECs, but not in ciliated cells and that TMEM16A protein gathered around mucin granules. The finding of the presence of TMEM16A in both the cell membrane and the cytoplasm raises the possibility that TMEM16A could be involved in the transport of intracellular mucin and extracellular mucin secretion.
So far, studies on the role of TMEM16A in mucus secretion have mainly focused on hydration of mucus, involving the relationship between Cl- secretion and viscous/elastic properties of mucus. Although TMEM16A has been reported as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells,25 a recent study in mice lacking the expression of TMEM16A (Tmem16a(-/-) mice) has demonstrated a defect in Ca2+-dependent Cl- secretion in native epithelia of these mice.31 Similarly, Ca2+-dependent Cl- transport is missing or largely reduced in isolated tracheal and colonic epithelia, and measurements of particle transport on the surface of tracheas ex vivo indicate largely reduced mucociliary clearance.31 Rock et al.23 have suggested that in Tmem16a(-/-) mice, TMEM16A-mediated Cl- secretion may be necessary for normal airway surface liquid homeostasis. They also stated that in trachea of these mice, there was a >60% reduction in purinoceptor (UTP)-regulated CaCC activity and that the residual CaCC activity appeared inadequate for normal airway hydration because Tmem16a(-/-) tracheas exhibited significant, neonatal, luminal mucus accumulation. Calcium-dependent chloride channel activity has also been shown to be increased by the treatment of bronchial epithelial cells with interleukin-4 (IL-4).21 Addition of UTP increases currents due to Cl- secretion. However, UTP-dependent Cl- currents are significantly reduced in cells transfected with anti-TMEM16A siRNA, with or without IL-4 treatment.21 Collectively, those studies have documented that TMEM16A plays an important role in the hydration of mucus, particularly by Cl- ion transport.
To date, little information is available on the effects of TMEM16A on the production and secretion of mucin in a Th2 inflammatory environment. Our study showed that the expressions of TMEM16A and MUC5AC proteins were significantly increased, as indicated by Western blot analysis and ELISA, in dissociated nasal polyp epithelium ex vivo and in ALI-cultured sinonasal epithelial cells from control subjects and patients with CRSwNP, after exposure to IL-13. Some researchers have reported that IL-13 can increase goblet cell density and that the elimination of IL-13 reverses the established goblet cell metaplasia into ciliated cells in airway epithelial cell culture.32 We found that the expressions of TMEM16A and MUC5AC were closely related to epithelial cell differentiation and that the lack of IL-13, i.e., elimination of the Th2 inflammatory environment, resulted in weak expressions of these proteins in ALI-cultured sinonasal epithelial cells. A previous study has shown that niflumic acid, a blocker of calcium-activated chloride channel (CLCA, former CaCC), effectively suppresses goblet cell hyperplasia, eosinophilic infiltration, and the expression of MUC5AC gene after instillation of IL-13 into mice airways, via the JAK2/STAT6 pathway.33 Although TMEM16A has drawn more attention as a CaCC, whether it involves mucin secretion in upper chronic inflammatory airways has not been investigated. A recent study has shown that IL-13 dramatically increases CaCC currents, as measured by the short circuit current (Isc) in the Ussing chamber, and TMEM16A mRNA in human bronchial epithelial cells.24 Our data suggest that T16Ainh-A01, a TMEM16A inhibitor, may suppress goblet cell differentiation induced by IL-13, decreasing the percentage of TMEM16A positive cells, the percentage of MUC5AC-positive cells, the percentage of cells coexpressing TMEM16A and MUC5AC, with subsequent reduction in the expression of TMEM16A protein and the secretion of MUC5AC. However, T16Ainh-A01 has no effect on mucin secretion in inactivated HNPECs in the absence of IL-13.
In summary, IL-13 increased the percentages of TMEM16A-positive cells, MUC5AC-positive cells, and cells coexpressing TMEM16A and MUC5AC, the expression of TMEM16A protein, and the secretion of MUC5ACin HNPECs. This study suggests that TMEM16A may play an important role in the modulation of mucin secretion in HNPECs induced by IL-13, and provide an insight into novel ways to improve abnormal mucus secretion in CRSwNP or other nasal inflammatory diseases associated with Th2 cytokines. However, as mucin secretion is a complex and delicate process, involving mucin granule production, movement of granules, fusion of granules and membrane, and opening of the granule with release of mucin onto the surface of the airways,34 future studies need to focus on molecular mechanisms underlying the role of TMEM16A in mucin production and secretion from airway epithelial cells induced by Th2 cytokines.
ACKNOWLEDGMENTS
This work was supported by grants from the Program for Changjiang Scholars and Innovative Research Team (IRT13082), the National Science Fund for Distinguished Young Scholars and for International Cooperation (81025007 and 81420108009), The twelfth five year science and technology support program (2014BAI07B04), Beijing Natural Science Foundation (7131006), Ministry of Health Foundation (201202005), the Capital Health Research and Development of Special (2011-1017-06), the Special Fund of Sanitation Elite Reconstruction of Beijing (2009-2-007), Beijing Science and Technology Program (Z121107009212032), and Beijing Health Bureau Program for high level talents (2011-3-043).
References
2. Drake-Lee AB, Lowe D, Swanston A, Grace A. Clinical profile and recurrence of nasal polyps. J Laryngol Otol. 1984; 98:783–793. PMID: 6470574.

3. Settipane GA, Klein DE, Settipane RJ. Nasal polyps. State of the art. Rhinol Suppl. 1991; 11:33–36. PMID: 1888555.
4. Ramanathan M Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008; 22:115–121. PMID: 18416964.

5. Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, et al. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013; 131:772–780. PMID: 23375208.

6. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289. PMID: 17002703.

7. Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008; 121:1435–1441. 1441.e1–1441.e3. PMID: 18423831.

8. Lu X, Wang N, Long XB, You XJ, Cui YH, Liu Z. The cytokine-driven regulation of secretoglobins in normal human upper airway and their expression, particularly that of uteroglobin-related protein 1, in chronic rhinosinusitis. Respir Res. 2011; 12:28. PMID: 21385388.

9. Ocampo C, Suh L, Kern R, Kato A, Conley D, Chandra R, et al. Levels of the cytokines IL-5, IL-13 and Rantes in nasal lavage fluids parallel the cytokine content of nasal polyps in patients with chronic rhinosinusitis with nasal polyps (CRSwNP). J Allergy Clin Immunol. 2013; 131:AB237.

10. Nabavi M, Arshi S, Bahrami A, Aryan Z, Bemanian MH, Esmaeilzadeh H, et al. Increased level of interleukin-13, but not interleukin-4 and interferon-γ in chronic rhinosinusitis with nasal polyps. Allergol Immunopathol (Madr). 2014; 42:465–471. PMID: 23969075.

11. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; 126:962–968. 968.e1–968.e6. PMID: 20810157.

12. Iwashita H, Fujimoto K, Morita S, Nakanishi A, Kubo K. Increased human Ca2+-activated Cl- channel 1 expression and mucus overproduction in airway epithelia of smokers and chronic obstructive pulmonary disease patients. Respir Res. 2012; 13:55. PMID: 22731784.

13. Huang F, Wong X, Jan LY. International Union of Basic and Clinical Pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol Rev. 2012; 64:1–15. PMID: 22090471.

14. Cunningham SA, Awayda MS, Bubien JK, Ismailov II, Arrate MP, Berdiev BK, et al. Cloning of an epithelial chloride channel from bovine trachea. J Biol Chem. 1995; 270:31016–31026. PMID: 8537359.

15. Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002; 99:4008–4013. PMID: 11904445.

16. Qu Z, Wei RW, Mann W, Hartzell HC. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl- currents. J Biol Chem. 2003; 278:49563–49572. PMID: 12939260.

17. Suzuki M, Mizuno A. A novel human Cl(-) channel family related to Drosophila flightless locus. J Biol Chem. 2004; 279:22461–22468. PMID: 15010458.

18. Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J. 2009; 97:3047–3053. PMID: 20006941.

19. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008; 134:1019–1029. PMID: 18805094.

20. Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008; 455:1210–1215. PMID: 18724360.

21. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008; 322:590–594. PMID: 18772398.

22. Tian Y, Kongsuphol P, Hug M, Ousingsawat J, Witzgall R, Schreiber R, et al. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011; 25:1058–1068. PMID: 21115851.

23. Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, et al. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl- secretory channel in mouse airways. J Biol Chem. 2009; 284:14875–14880. PMID: 19363029.

24. Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci U S A. 2012; 109:16354–16359. PMID: 22988107.

25. Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011; 286:2365–2374. PMID: 21084298.

26. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12. PMID: 22469599.

27. Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol. 2002; 27:536–541. PMID: 12397012.

28. Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. 2011; 41:1747–1756. PMID: 22092504.

29. Patou J, Gevaert P, Van Zele T, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008; 121:110–115. PMID: 17980412.

30. Scudieri P, Caci E, Bruno S, Ferrera L, Schiavon M, Sondo E, et al. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol. 2012; 590:6141–6155. PMID: 22988141.

31. Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem. 2009; 284:28698–28703. PMID: 19679661.

32. Kondo M, Tamaoki J, Takeyama K, Isono K, Kawatani K, Izumo T, et al. Elimination of IL-13 reverses established goblet cell metaplasia into ciliated epithelia in airway epithelial cell culture. Allergol Int. 2006; 55:329–336. PMID: 17075276.

33. Nakano T, Inoue H, Fukuyama S, Matsumoto K, Matsumura M, Tsuda M, et al. Niflumic acid suppresses interleukin-13-induced asthma phenotypes. Am J Respir Crit Care Med. 2006; 173:1216–1221. PMID: 16528019.

Fig. 1
Immunohistochemical staining for TMEM16A and MUC5AC in human sinonasal epithelium: TMEM16A and MUC5AC proteins in human nasal polyp epithelium (HNPE group) (A, B) and normal sinonasal epithelium (control group) (C, D). Tissues were stained with anti-TMEM16A (1:200) and anti-MUC5AC (1:500). Arrowheads show TMEM16A-positive cells, and arrows show MUC5AC-positive cells. The images were observed with a 40× objective.
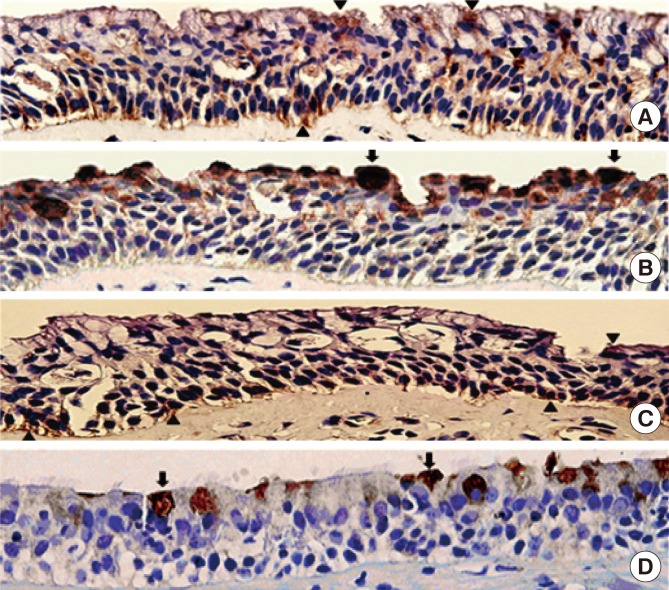
Fig. 2
Expressions of TMEM16A and MUC5AC proteins in dissociated human sinonasal epithelium. (A) The expression of TMEM16A protein was detected by Western blotting in dissociated normal sinonasal epithelium (control group) and nasal polyp epithelium (HNPE group). (B) Comparison of the expression of TMEM16A protein by densitometric analysis between the control and HNPE groups. Each bar represents the relative density of TMEM16A band normalized to β-actin band for each sample (n=6, ***P<0.001). (C) Measurement of MUC5AC by ELISA from supernatants of nasal tissue homogenate in control and HNPE groups (n=6, ***P<0.001).

Fig. 3
Coexpression of TMEM16A and MUC5AC in ALI-cultured HNPECs. Confocal microscopy xy images of ALI-cultured HNPECs without IL-13 (A) and with IL-13 treatment (10 ng/mL, 14 days) (B) showing over lapping immunoreactivity for TMEM16A (red) and MUC5AC (green). Nuclei stained with DAPI appear blue. The images were taken with a 40× objective. Scale bars: 50 µm.
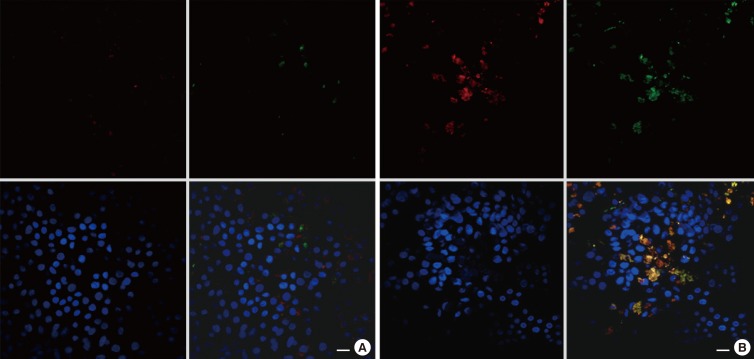
Fig. 4
Expression of TMEM16A in non-ciliated cells. Confocal microscopy xy images of ALI-cultured HNPECs without IL-13 (A) and with IL-13 (10 ng/mL, 14 days) (B) showing immunoreactivity for TMEM16A (red) and acetylated-tubulin (green). Nuclei stained with DAPI appear blue. The 2 images show that TMEM16A and acetylated-tubulin staining appears on different epithelial cells. The images were taken with a 40× objective. Scale bars: 50 µm.

Fig. 5
Increased expression of TMEM16A and secretion of MUC5AC in ALI-cultured HNPECs with IL-13. (A) The percentage of TMEM16A-positive cells, MUC5AC-positive cells, and cells coexpressing MUC5AC and TMEM16A in ALI-cultured HSNECs (control group) and HNPECs incubated with or without IL-13 (10 ng/ml) for 14 days (n=6, ***P<0.001). (B) Detection of TMEM16A protein by Western blot in lysates from HNPECs and control cells incubated with or without IL-13 (10 ng/mL) for 14 days. (C) Comparison of the expression of TMEM16A protein by densitometric analysis. Each bar reports the relative density of TMEM16A band normalized to β-actin band for each sample (n=6, ***P<0.001). (D) Detection of MUC5AC by ELISA in apical washings from HNPECs and control cells incubated with or without IL-13 (10 ng/mL) for 14 days (n=6, ***P<0.001).
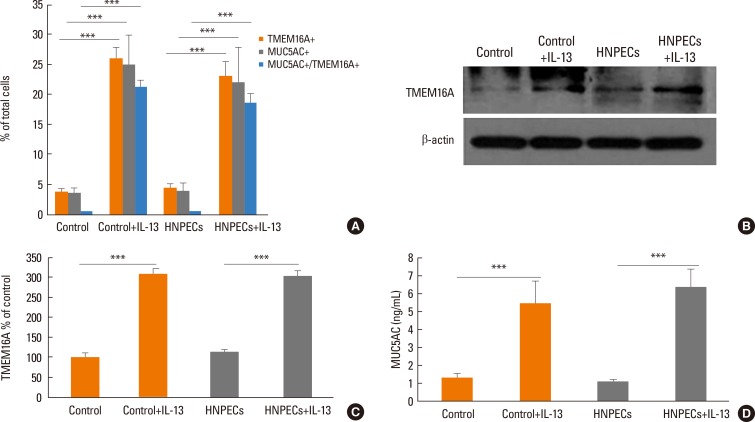
Fig. 6
Decreased expression of TMEM16A and secretion of MUC5AC in ALI-cultured HNPECs by the TMEM16A inhibitor T16Ainh-A01. (A) The percentage of TMEM16A-positive cells, MUC5AC-positive cells, and cells coexpressing MUC5AC and TMEM16A in ALI-cultured HNPECs incubated with or without IL-13 (10 ng/mL) + T16Ainh-A01(10 µM) for 14 days (n=6, *P<0.05, ***P<0.001). (B) Western blot analysis of TMEM16A protein in lysates from HNPECs incubated with or without IL-13 (10 ng/mL) + T16Ainh-A01(10 µM) for 14 days. (C) Comparison of the expression of TMEM16A protein by densitometric analysis. Each bar shows the relative density of TMEM16A band normalized to β-actin band for each sample (n=6, ***P<0.001). (D) Detection of MUC5AC by ELISA in apical washings from HNPECs incubated with or without IL-13 (10 ng/mL) + T16Ainh-A01 (10 µM) for 14 days (n=6, *P<0.05, **P<0.01, ***P<0.001).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download