Abstract
Purpose
Methods
Results
Figures and Tables
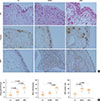 | Fig. 1The distribution of eosinophlis, neutrophils, and mast cells in polyp tissues of atopic and nonatopic CRSwNP patients. (A) Representative HE stainings (indicating eosinophils), and IHC stainings of MPO (indicating neutrophils) and Tryptase (indicating mast cells) in atopic and nonatopic polyp tissues and normal controls. (B) The mean numbers of eosinophlis, neutrophils, and mast cells in atopic and nonatopic polyp tissues and normal controls. Co, control; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps. |
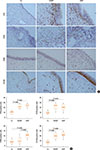 | Fig. 2The distribution of T cells, B cells, plasma cells, and macrophages in polyp tissues of atopic and nonatopic CRSwNP patients. (A) Representative IHC stainings of CD4 (indicating T cells), CD20 (indicating B cells), CD138 (indicating plasma cells), and CD68 (indicating macrophages) in atopic and nonatopic polyp tissues and normal controls. (B) The mean numbers of T cells, B cells, plasma cells, and macrophages in atopic and nonatopic polyp tissues and normal controls. Co, control; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps. |
 | Fig. 3The mRNA expressions of FOXP3, T-bet, GATA3, and RORc in polyp tissues of atopic and nonatopic CRSwNP patients and normal controls. Co, control; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps. |
 | Fig. 4The concentrations of total IgE, SAE-specific IgE, ECP, and MPO in polyp tissues of atopic and nonatopic CRSwNP patients and normal controls. Co, control; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps. |
 | Fig. 5The concentrations of IFN-γ, IL-5, IL-17A, IL-1β, IL-2sRα, IL-6, IL-8, IL-10, TGF-β1, TNF-α, and IL-2 in atopic and nonatopic polyp tissues and normal controls. Co, control; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps. |
 | Fig. 6The concentrations of IFN-γ, IL-5, IL-17A, IL-1β, IL-2sRα, IL-6, IL-8, IL-10, TGF-β1, TNF-α, and IL-2 in stimulated atopic and nonatopic polyp tissues after anti-IgE stimulation. RPMI, Roswell Park Memorial Institute; Co, control; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps. |
Table 1
Patient characteristics and symptom scores

Data are expressed as medians and interquartile ranges. The level of significance (P) was obtained by means of ANOVA or Likelihood Ratio (marked with*). A P value of <0.05 was considered statistically significant. For comparisons of continuous variables between the 2 groups, the Mann-Whitney U test was performed (†Control vs NANP; ‡Control vs ANP; §NANP vs ANP).
NANP, nonatopic nasal polyps; ANP, atopic nasal polyps; VAS, visual analogue scales.
Table 2
Analysis of peripheral blood cells

Data are expressed as medians and interquartile ranges.
*Control vs NANP; †Control vs ANP; ‡NANP vs ANP (the Mann-Whitney U 2-tailed test was used for between-group comparison, with significance level set at P<0.05).
NANP, nonatopic nasal polyps; ANP, atopic nasal polyps; VAS, visual analogue scales.
Table 3
Levels of local IgE in polyp and control nasal tissues

Data are expressed as medians and interquartile ranges.
*Control vs NANP; †Control vs ANP; ‡NANP vs ANP (the Mann-Whitney U 2-tailed test was used for between-group comparison, with significance level set at P<0.05).
SIgEg6, Timothy grass pollen-specific IgE; SIgEd1, Dermatophagoides pteronyssinus-specific IgE; NANP, nonatopic nasal polyps; ANP, atopic nasal polyps; VAS, visual analogue scales.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download