Abstract
Immunoglobulin E (IgE) can be highly elevated in the airway mucosa independently of IgE serum levels and atopic status. Mostly, systemic markers are assessed to investigate inflammation in airway disease for research or clinical practice. A more accurate but more cumbersome approach to determine inflammation at the target organ would be to evaluate markers locally. We review evidence for local production of IgE in allergic rhinitis (AR) and chronic rhinosinusitis with nasal polyps (CRSwNP). Diagnostic and therapeutic consequences in clinical practice are discussed. We describe that the airway mucosa has the intrinsic capability to produce IgE. Moreover, not only do IgE-positive B cells reside within the mucosa, but all tools are present locally for affinity maturation by somatic hypermutation (SHM), clonal expansion, and class switch recombination to IgE. Recognizing local IgE in the absence of systemic IgE has diagnostic and therapeutic consequences. Therefore, we emphasize the importance of local IgE in patients with a history of AR or CRSwNP.
Immunoglobulin E (IgE) is a major contributing factor in multiple airway diseases, including allergic rhinitis (AR) and chronic rhinosinusitis with nasal polyposis (CRSwNP). However, measuring IgE by classical systemic tests fails to give an adequate idea of local IgE in the target organ, the nose.1,2,3,4 In this review, we summarize the evidence of local production of IgE in sinonasal diseases, and clinical implications will be discussed.
Diagnostic tools in rhinitis may not be sufficient to differentiate between allergic, non-allergic, and local allergic rhinitis (LAR), as local IgE normally is not measured.
In chronic rhinosinusitis, different disease subgroups exist5 with their inherent pathomechanisms, and it is challenging to find good markers to further categorize the nasal-polyposis population. It would be especially interesting to determine the endotype in which IgE, whether or not systemic, is crucial in the pathogenesis. The response to targeted therapy as anti-IgE can be predicted.
Mature naïve B cells encounter antigen processed and presented by dendritic cells in peripheral lymphoid organs. They become activated after interaction with T cells specific for an incoming antigen. After activation on the boundary between B-cell follicles and T-cell zones, the B cells have 2 options. They migrate to the follicle, proliferate, and form germinal centers, or they migrate to an extra-follicular region, proliferate, and differentiate into short-lived plasma cells. B cells in the germinal center undergo antibody affinity maturation by means of somatic hypermutation (SHM), class switch recombination (CSR), clonal expansion, and selection.
SHM and CSR are necessary to create enormous diversity found in the antibody and T-cell receptor repertoires required for an effective immune response. As mentioned before, these reactions generally take place within germinal centers, which are typically located in secondary lymphoid tissues, such as tonsil tissue, lymph nodes, and the spleen. SHM is a modification of the genome sequence in somatic cells by substitution of a single base in variable regions of Immunoglobulin (Ig) genes in B cells. CSR in the Ig heavy chain gene locus of the constant region is necessary to class switch from IgM, IgG, or IgA to IgE, resulting in B cells expressing IgE. Both SHM and CSR are initiated by activation-induced cytidine deaminase (AID),6 and thus this molecule can be used as a marker for these processes. When a mature B cell alters its receptor in response to antigenic stimulation, this is called receptor revision (RR), initiated by recombination- activating gene products (RAG1 and RAG2).
Signals from T helper cells are mandatory for CSR to IgE+ cells, namely interleukin (IL) 4 and IL13, and the ligation between CD40 on B cells and CD40ligand on T cells.7 After binding of the promotor Iε, the production of germline transcripts (εGLT) is intitiated. This precedes IgE class switching and recombination of the heavy chain by AID. The outcome is a mature ε chain mRNA and a circular fragment of DNA that is looped out, known as a 'switch circle.' Ergo switch circles can be used as a marker of ongoing CSR in B cells.
Isotype-switched B cells that leave the germinal center reaction become either memory B cells or long-lived plasma cells. Memory B cells divide, whereas long-lived plasma cells do not self-renew. Memory B cells secrete little Ig, but rapidly provide antigen-specific antibody-secreting plasma cells upon antigen recall. Long-lived plasma cells provide long-term maintenance of antigen-specific antibody titers. This is likely the case for IgE as well.8 Both B memory cells and long-lived plasma cells are the cellular source of IgE memory, and they ensure humoral memory. In a murine model, the results of the group of Erazo9 suggest that IgE+ cells that exit the germinal center reaction preferentially develop into plasma cells. The differentiation of B cells into plasma cells is directed by B cell-activating factor of the TNF-family (BAFF) and B-lymphocyte-induced maturation protein (BLIMP).
Rhinitis is traditionally categorized as allergic, infectious, or non-infectious non-allergic rhinitis (NINAR). NINAR is diagnosed by exclusion,10 meaning that this category includes a heterogeneous group of rhinitis patients with a poorly defined pathogenesis. Mostly, no etiology is found, and this subgroup is known as idiopathic rhinitis.
IgE is one of the most important markers of allergy, as it is the major contributing factor in most types of allergic disease.11 Traditionally, the distinction between allergic and non-allergic rhinitis is based on skin prick tests and serum IgE analysis. However, in a subgroup of patients with a typical history suggestive of allergy, these tests are negative, but measurement of local IgE can show elevated levels. This observation suggests that a local form of rhinitis may exist, known as 'LAR'.
Respiratory mucosa is a site of IgE induction during allergic airway inflammation. The group of Eckl-Dorna12 investigated the role of blood-derived plasma cells and B cells in the production of allergen-specific IgE. In a series of cell separation experiments performed by negative depletion and positive selection, they found that the majority of circulating specific IgE antibodies is not derived from IgE-producing cells in the blood. This result suggests that the production at the target organ is the probable source.12 Serum IgE in AR might thus be a result of spill over rather than the reverse; nearly all of the serum IgE may be derived from mucosal sites.
Conventionally, an immune response is primed in germinal centers within lymph nodes, whereas the effector function of antibodies is peripheral, e.g. mucosal. In AR, however, all events may occur peripherally.13 Localized IgE-mediated inflammation may be suspected in a small subgroup of the idiopathic rhinitis group with negative skin prick testing, where allergen-specific IgE is measurable in nasal secretions of patients.14 Also, studies have proven that the localized cellular pathogenesis is analogous to that in patients with AR, suggesting that these 'non-allergic' subjects are in fact allergic.
In AR, IgE-positive B cells would reside in the nasal mucosa, as local IgE production has been perceived in vitro15 and in vivo.14,16,17 The possibility that IgE is produced locally in the target organ was first proposed after an experiment in children with AR to house dust mite and asthma. IgE was measured in the serum and in nasal secretions in steady state, and kinetic studies were added. It was seen that after re-exposure, local IgE increased more rapidly than serum IgE.18 In another experiment, levels of specific IgE measured in nasal secretions exceed levels measured in serum of patients diagnosed with AR to cedar pollen.19 Furthermore, in cases where no serum specific IgE is measurable, elevated levels are found in nasal secretions. This was demonstrated for HDM-specific IgE16,20 and for specific IgE to grass/olive pollens14 in non-allergic patients with positive nasal provocation tests.
Kleinjan et al.17 provided evidence for local production of specific IgE, as IgE-positive mast cells, plasma cells, and B cells11 are found in inferior turbinate tissue from patients with AR. The presence of long-lived plasma cells in the mucosa of subjects with AR was also suggested by Smurthwaite et al.21 who demonstrated persistent IgE synthesis between seasons in explants of hay fever patients. Ex vivo stimulation of tissue obtained from allergic patients leads to an increase in IgE, meaning that all is available locally to produce IgE.13 Cameron et al.22 demonstrated elevated levels of IL4 in AR, meaning the nasal mucosa in AR is a favorable environment for CSR. Hence, local production and release of IL4 and IL13 by T cells and mast cells may regulate the local IgE production.23 These cells also carry the CD40 ligand necessary for DNA recombination and IgE synthesis.23 Regarding functionality, Pawankar et al.15 demonstrated up-regulation of FcεRI on mast cells in response to locally produced specific IgE. Subsequently to the increased IgE cross-linking, more mast cells are activated and degranulate, promoting allergic reactions.
As previously discussed, evidence points at the local synthesis and secretion of IgE. Does CSR to IgE+ B cells and affinity maturation occur in distant lymphoid tissues before migrating to the target organ or do they first migrate and only then class switch?
In human nasal mucosa of allergic individuals, GC formation has not yet been demonstrated,24 although SH and CSR to IgE have been described.3,4,9,24,25 In an ex vivo experiment with nasal mucosa from grass pollen-sensitized subjects, class switching is found upon allergen stimulation.4 The group of Coker26 detected local bias to the VH5 germline gene family, while no VH5 overexpression was detected in the blood. Furthermore, AID is continuously expressed, representing a fundamental aberration in the mucosa of AR patients.4,27 Next to AR, local IgE formation is also present in CRSwNP. IgE in nasal polyps is polyclonal, whereas IgE in AR is monoclonal or oligoclonal. In contrast to AR, germinal center formation has been documented in nasal polyps,28 autoimmune diseases,27 and lower airways.13
To understand the role of IgE in allergic and 'non-allergic' rhinitis, knowledge of pathomechanisms is essential. Allergic inflammation typically comprises an early and a late phase organized by structural epithelial cells, residential mast cells, and infiltrating eosinophils/basophils/T cells. Cytokines released from mast cells and T cells mediate local IgE production by B cells.
Epithelial cells are not merely functional as a barrier, but upon activation they can also release immunomodulatory substances that regulate Th2 cytokine response, including eicosanoids, endopeptidases, cytokines (thymic stromal lymphopoietin [TSLP], IL25, and IL33), and chemokines. Epithelial cells can be activated by an IgE-mediated mechanism.
The activation of mast cells is crucial in the immediate response, and activation by antigen cross-linking of IgE is a well-known mechanism. Sensitized mast cells have both high and low affinity receptors for IgE on their surface, the abg2 tetramer FcεRI and the ag2 trimer FcεRII, respectively. The latter is also termed CD23 receptor and found on a broad range of cells.30 These receptors bind IgE, and upon cross-linking the mast cell degranulates and releases different mediators like histamine, tryptase, newly synthesized lipid mediators, cytokines, and chemokines. Histamine, leukotrienes, and prostaglandins cause clinical symptoms, such as sneezing, running nose, and nasal obstruction.
Basophils are phenotypically similar to mast cells, as they are granulocytes containing histamine and expressing FcεRI. Thus, upon cross-linking with specific IgE, they release histamine. However, basophils can migrate to lymphoid tissues, while mast cells cannot. It is suggested that effector functions of basophils are heterogeneous according to the eliciting factor. IL3-elicited basophils would be highly responsive to IgE, conversely to TSLP-elicited basophils that would be non-responsive to IgE.31
The release of cytokines and chemokines by mast cells in the immediate phase response, which starts within minutes and lasts for 2-3 hours, is important for the subsequent late phase response. The late phase response starts 4-6 hours after stimulation and lasts for 18-24 hours. An infiltrate containing T helper 2 (Th2) cells, eosinophils, and basophils is characteristic of this phase. Th2 cells are important for the production of key cytokines, including IL4, IL5, IL9, and IL13. These cytokines are essential for antibody class switching, regulation of local and systemic IgE synthesis, recruitment of eosinophils, basophils, and mast cells, and survival of eosinophils. It is mostly assumed that eosinophils differentiate within the bone marrow in response to eosinophilopoietins. In AR, however, a subset of eosinophils would differentiate within the nasal mucosa in a highly IL5-dependent manner.32 Langerhans cells are dendritic cells of the skin and mucosa. They are professional antigen-presenting cells (APCs), which process aeroallergens deposited on the mucosa and subsequently present the antigens to T cells. Th2 cells release their mediators upon recognition of antigen presented by APCs. The Th2 cytokines IL4, IL13, and CD40L induce selective somatic recombination of Ig heavy chain regions in B cells before maturation into IgE-producing plasma cells.
In 'non-allergic' rhinitis, no systemic markers of allergy can be detected. These patients could however still be allergic in the case of LAR, although most cases are truly non-allergic. Here, a broad differential diagnosis has to be made. Endonasal infections are a common cause of non-allergic rhinitis, and complaints can persist long after resolution of the infection. Nonspecific exogenous irritants, such as tobacco smoke, pollution, dust, smog, perfumes, solvents, and occupational irritants, could also trigger mucosal inflammation. Furthermore, rhinitis can be elicited by foods and beverages, namely 'gustatory rhinitis' by certain oral medications, or by overuse of decongestant nasal sprays, called 'rhinitis medicamentosa.' Changes in hormones due to pregnancy or hypothyroidism, for example, could result in rhinitis. In the elderly, watery rhinitis or geriatric rhinitis is often seen. Non-IgE-mediated hypersensitivity can also explain the discrepancy between IgE testing results and clinical findings, for example, T-cell mediated delayed type hypersensitivity and activation of mast cells by Ig free light chains (FLCs).33,34
Evidence increases for the possibility that a T cell-mediated inflammatory reaction to common antigens sustains asthma and AR, especially when atopic dermatitis is identified in the history.34 Here again, negative skin prick test (SPT) and RAST can be expected.
Ig FLC could serve as an alternative for IgE in eliciting inflammation in allergic disorders, including food allergy, atopic dermatitis, rhinitis, and asthma.35 FLCs can cause immediate hypersensitivity through mast cell activation, and they are found in both atopic and non-atopic rhinitis.35 Moreover, Powe et al.33 demonstrated a link between FLC immunoglobulins and mast cells in the nasal mucosa, which suggests an association between Ig FLC and mast cell-mediated nasal hyperreactivity. The role of FLC in AR is not clear, and further studies are necessary to investigate the function of FLC in local hypersensitivity.
Currently, the diagnostic workup of AR must consist of a history of the patient, with most important differentiation between intermittent and persistent symptoms and between mild and moderate to severe AR following the ARIA guidelines. In addition, clinical examination along with anterior rhinoscopy and nasoendoscopy is required. At last, allergen extract-based IgE tests are indispensable, such as SPT and the measurement of specific IgE antibodies in serum. Though these conventional tests are expected to be negative in cases where IgE is only situated in the nasal mucosa and those where rhinitis is induced by non-IgE-mediated mechanisms. These patients are classified as 'non-allergic,' although additional testing for local IgE may reveal a local allergic response in a subgroup of patients. In these cases where history is truly suggestive of AR and currently used tests are negative, it has to be questioned whether measurement of systemic IgE levels is sufficient for the diagnostic workup.
Additional testing by means of nasal provocation, as proposed by Rondón et al.36 or measurement of local IgE in the nasal mucosa can be considered to reveal LAR. However, it is currently unclear how large this subgroup is; findings from our clinic/group point to rather small numbers.37 Methods to measure local mediators have not yet been standardized, and this will be discussed further in this review. To determine whether T cell-mediated, delayed hypersensitivity lies at the basis of the complaints, an Atopy Patch Test can be useful34; however, the relevance of this test in AR has not yet been established.
The correct diagnosis and knowledge of pathophysiology are essential in establishing the best treatment for a patient.
Mast cells are crucial in allergic inflammation, and different treatment options aim to block their effect. Traditionally, topical corticosteroids and antihistamines are prescribed.
Avoidance of allergens if possible is an important step, for instance, in AR to mold or cat allergens. In most cases, however, it seems that this is hardly effective or impossible, such as in the case of AR to grass pollen or house dust mite. Only when it is known to which allergen the patient is sensitized, avoidance of allergen is possible, and/or immunotherapy can be considered. Recent diagnostic tests based on recombinant allergens, epitopes, and peptides allow more precise diagnosis of allergy. New immunotherapy strategies that would be more effective and safer than conventional allergy vaccines aim to suppress IgE-mediated inflammation. T-cell tolerance can be induced by administration of peptides containing T-cell epitopes, carrier-bound allergen peptides, or recombinant hypoallergens.
Targeted treatments using antibodies are developing. In allergic diseases, such as AR, asthma, food allergy, and allergic dermatitis, anti-IgE or omalizumab has been investigated. This humanized monoclonal antibody targets the Cε3 domain of IgE, reducing free IgE by forming a biological inert molecule. Furthermore, omalizumab down-regulates FcεRI on effector cells, and it would also decrease IgE synthesis by inducing an anergic state in IgE+ B cells.38 Omalizumab has a good clinical effect in allergic diseases39 that lasts for about 4 months, and it has a low incidence of side effects.40 This treatment is, in theory, an option in AR. However, the high cost-effectiveness ratios41 result in restriction of indications. Theoretically, rituximab or anti-CD20 could also be an option to inhibit IgE-producing cells.
To conclude, allergen avoidance (if possible), local corticoids, antihistaminic drugs, and leukotriene receptor antagonists are the first-line treatment options. Immunotherapy can be considered in certain cases where the conventional treatment is not sufficiently effective. In this way, a good control of symptoms is achievable and omalizumab or rituximab are not indicated for AR as such.
The term chronic sinusitis covers a wide range of sinus diseases, in which the mucosa is inflamed with symptoms on most days lasting at least 12 weeks without complete resolution. In the pathophysiology, intrinsic (for example genetic) and extrinsic (for example microbial, environmental, and iatrogenic) factors may be involved, which can act locally or systemically.
Two phenotypes are currently distinguished, namely chronic rhinosinusitis without nasal polyposis (CRSsNP) and CRSwNP. However, some authors believe that different disease entities classified as chronic sinusitis are a continuum of the same disease. These phenotypes show considerable overlap in symptoms, but can be differentiated from each other with reasonable certainty by endoscopy.
The definition 'chronic sinusitis' suggests a single clinical entity, but in reality it represents multiple overlapping entities with different inflammation patterns.
In CRSsNP, mostly Th1-skewed neutrophilic inflammation is found with elevated levels of interferon-γ (IFNγ) and transforming growth factor-β (TGFβ), whereas inflammation in CRSwNP is often Th2-skewed eosinophilic inflammation with elevated levels of IL5 and IgE.5 However, patients with CRSwNP also form a heterogeneous group with different endotypes, meaning that inflammation patterns and T helper subsets vary.
In the Western countries, the majority of nasal polyps is characterized by Th2-skewed inflammation, dominated by cytokines that promote production of IgE and eosinophilic inflammation. IL4 stimulates proliferation and differentiation in Th2 cells and mediates CSR to IgE by stimulating the synthesis of ε-germline gene transcript (GLT). IL5 is elevated in CRSwNP and remarkably increased in CRSwNP accompanied by non-allergic asthma and aspirin sensitivity.42 IL5 mediates eosinophil survival, differentiation, and recruitment to the nasal mucosa, and causes secretion of eosinophil cationic protein (ECP).43 ECP is an indicator of eosinophilic activation, and local ECP correlates with local IgE.44 The local inflammatory pattern could be related to the type of bacterial colonization. Colonization with Gram-positive bacteria, for example, Staphylococcus aureus (SA) is common in individuals with nasal polyps characterized by elevated IL5, ECP, and total IgE. In Asian populations, the inflammation in CRSwNP is frequently predominantly neutrophilic.45 This pattern is associated with greater Gram-negative bacterial colonization. Patients with nasal polyps and cystic fibrosis have Th1/Th17-skewed neutrophilic inflammation with highly increased expression of IL17 and IFNγ.5
Biochemical markers that could be used to differentiate between CRSsNP and CRSwNP in Caucasians are ECP, IL5, and IgE. Markers to differentiate between CRSwNP and cystic fibrosis with nasal polyps also include IL8, IL17 and MPO.5
Total IgE is often highly increased in nasal polyp mucosa, and IgE specific to staphylococcal enterotoxins can be present independently of serum total/specific IgE.46 The presence of plasma cells in the mucosa insinuates local production of IgE, which is polyclonal and functional.47
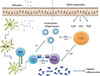
In atopic nasal polyp patients, local IgE production can be the effect of stimulation with allergens.48 However, local hyperimmunoglobulinemia is also present in non-atopic patients, meaning that elevated IgE levels result from other pathways as well. The cytokines IL25 and IL33 can induce IgE-mediated inflammation by stimulating a non-T cell source to produce IL4, IL5, and IL13, namely innate lymphoid cells (ILC)49 (Fig. 1). A role of mast cells in enhancing eosinophilic inflammation in chronic rhinosinusitis is suggested. Ex vivo experiments demonstrate that activation of IgE cross-linking in CRSwNP47 and FLCs present in nasal polyps could mediate local immune responses.50 FLC concentrations correlate with IL5, IL6, and local IgE. Furthermore, a decrease in local FLCs is seen after treatment with anti-IL5, presuming that IL5 creates an environment that favors FLC production.50 Next to IgE and FLC, locally produced IgA51 could also be involved in the activation of mast cells and eosinophils. The role of IgA is not clear, but elevated levels of IgA are often found in patients with chronic mucosal inflammation. In CRSwNP as well, elevated levels of IgA is found in tissue homogenates,51 although this could be explained by a decreased translocation of IgA from the epithelium of the lamina propria into nasal secretions.52
As mentioned earlier, it is generally believed that GC are situated in lymphoid tissues, meaning that affinity maturation by SHM and CSR takes place in these lymphoid tissues.
The involvement of local IgE production has been investigated by comparing key markers of Th2 inflammation and GC reactions in nasal polyp tissue versus control tissue. We measured elevated levels of IL4, ε-GLT, ε-mRNA, and local IgE, which all point at local CSR.
Enhanced differentiation of B cells into plasma cells in nasal polyps can be concluded from the increased number of plasma cells and ratio of plasma cells to B cells, together with the elevated BAFF levels. The up-regulation of RAG1/RAG2 points at local RR. The presence of switch circle transcripts points at ongoing local CSR. To conclude, our group was the first to reveal formation on GC-like structures in polyps with local RR, class switching to IgE, and B-cell differentiation into IgE-secreting plasma cells.1 Because T follicular helper (Tfh) cells and IL21 produced by these cells are important in regulating B cells in GC and lymphoid accumulations are found in nasal polyps, a new study was conducted. Here, IL21 and IL21- positive T helper cells were measured in nasal tissues of CRSwNP, CRSsNP, and controls. Elevated levels of IL21 and IL21-positive T helper cells were found in CRSwNP, and after stimulation with staphylococcal enterotoxin (SE) B during 24 hours a significant increase of IL21 in the CRSwNP was seen. In the same study, high expression of B lymphocyte-induced maturation protein-1 (Blimp-1) and B-cell lymphoma-6 (Bcl-6) was found. These molecules are antagonists with Bcl-6 being the regulator of Tfh cell differentiation and Blimp-1 suppressing generation of Tfh cells. The authors suggest that both mediators regulate differentiation of T and B cells in nasal polyp tissue. It is concluded that IL21 produced by Tfh cells stimulates GC formation in CRSwNP (unpublished data). The group of Mechtcheriakova53 used CRSwNP as a model to study AID-associated responses in diseases characterized by chronic inflammation. In nasal polyp tissue, they detected functional AID-positive ectopic follicular structures by real-time PCR analysis and immunostaining. No AID mRNA could be detected in the control CRSsNP tissue. The transcription of IgE and/or IgG was positively correlated with the expression of AID mRNA. These results indicate that CSR and SHM can take place locally in the airways at sites of chronic inflammation.
Local IgE in CRSwNP is polyclonal and associated with the presence of IgE antibodies to SEs.28,47 SA is one of the most frequently found bacteria in CRSwNP. Colonization with SA is significantly more prevalent in the population with nasal polyps compared to controls. Colonization is present in 27.3% of CRSsNP patients, 33% of control subjects, 60% of patients with CRSwNP and as high as 87% in the subgroup with asthma and aspirin intolerance, based on culture data.54 An increased colonization rate of SA was demonstrated in patients with CRSwNP (63.6%), but not in those with CRSsNP (27.3%) and control subjects (33%).55
Immune response rates to enterotoxines released by SA follow the same trend, with SE-specific IgE present in 28% of CRSwNP and up to 80% in the subgroup with aspirin-exacerbated respiratory disease.55 SE contributes to nasal polyp formation in both atopic and non-atopic patients,46,56 since these enterotoxins have the ability to induce enormous activation of lymphocytes without undergoing processing by APCs. Consequently, severe eosinophilic inflammation arises, and polyclonal IgE and IgG/IgG4 are produced by B cells.51 Products of viral or bacterial microorganisms with this feature are identified as superantigens.
The activation of T- and B-cells by T-and B-cell superantigens, respectively, is nonspecific and polyclonal, and leads to massive cytokine release. This resultant polyclonal IgE saturates the receptors on local tissue mast cells, reducing the effect of specific IgE and the allergic response. Superantigens skew the inflammation toward Th2-mediated inflammation with increased levels of ECP, IL5, and IgE, or a pre-existing Th2 milieu might facilitate the persistence of SA. Moreover, macrophages are alternatively activated, causing deficient phagocytosis of SA in CRSwNP.57 Detection of IgE specific to SE has been demonstrated for tissue homogenates, but rarely in serum of subjects without comorbid asthma. Moreover, the levels of IgE specific to SEA and SEB detected in tissue homogenates correlate with levels of ECP and IL5, and thus eosinophilic inflammation.58
Eosinophilic inflammation with elevated IgE found in nasal polyps suggests an atopic background, although atopy has no impact on tissue inflammatory patterns in Caucasian polyps. Epidemiological data support this notion, as CRSwNP is as prevalent in atopic patients as in non-atopic patients. Suh et al.59 compared total IgE and HDM-specific IgE in nasal polyp tissue from patients with strong or weak systemic hypersensitivity, and controls. They found a high IgE-level in both atopic groups, suggesting local IgE production definitely in patients with weak systemic atopy. In 2010, Sheahan et al.60 found local antigen-specific IgE in 57% of non-atopic nasal polyp tissue, supporting the presence of local IgE to both seasonal and perennial allergens.
CRSwNP is often associated with asthma and/or aspirin intolerance. CRSwNP and asthma share certain characteristics and often coexist, leading to the assumption that they could both be an expression of the same Th2-mediated disease. Importantly, there is evidence for local IgE synthesis in the bronchial mucosa of atopic and non-atopic asthmatics.29 The overexpression of IgE and IL5 in nasal polyps of the Th2 subtype of CRSwNP is, next to the presence of SE-specific IgE, associated with an increased risk of asthma.42 In patients with nasal polyps and asthma, local IgE is highly elevated as compared to controls, CRSsNP, and CRSwNP without asthma (Fig. 2). Sensitization to SE is frequent, with prevalence of positive serum SE-specific IgE of 29.3% in the European general population and 27% in the Korean adult population.61 Sensitization is more common in smokers, males, and persons sensitized to aeroallergens and of advanced age.61 Furthermore, serum SE-specific IgE is associated with adult-onset asthma61 and could be used as a biomarker.
Diagnostics are insufficient if solely based on clinical and radiographic evaluation of the paranasal sinuses. Differentiation between Th2-biased CRSwNP with high local IgE and that which is not Th2-biased is relevant in clinical practice and of prognostic value, as especially the eosinophilic type is hard to treat and has the reputation to relapse. Moreover, Th2-skewed inflammation involves an increased level of IL5, and it has been demonstrated that this cytokine is related to the development of asthma.42 Determining the presence of local SE-specific IgE is also important prognostically, as it would be linked to the development of asthma as well. Measuring markers like IgE in mucosal tissue is the most direct investigation of inflammation in the nose. In practice, however, collection of tissue is only reasonable when the patient needs to undergo surgery, as sinus mucosa biopsy is an invasive method.
Assuming that protein levels in nasal secretions correlate with those in the underlying tissue, an alternative is to measure local markers in nasal secretions. Several techniques are described to collect secretions that are all less invasive than biopsy. Nasal lavage and absorption techniques by placing filter papers (cellulose sheets) or foam in the nasal cavity are good options. The foam or filter paper equilibrates with the ambient fluid and is then squeezed by centrifugation.
At last, analogue to diagnostic techniques in AR, nasal provocation could be a diagnostic tool in CRSwNP. However, converse to AR, a study conducted by Calus et al.62 made it clear that this technique has little diagnostic value in CRSwNP.
Currently, the treatment of CRSwNP consists of medical treatment, but in most cases additional treatment by endoscopic sinus surgery is necessary. However, CRSwNP is a chronic disease with high relapse rates in certain endotypes. New treatment options are emerging to better control the disease, namely antibody treatments. However, given the cost it is likely that these tailored treatments will be reserved for patients with severe airway disease refractory to conventional treatment.
Medical treatment of CRSwNP often requires a combination of saline irrigation, topical nasal steroids/oral steroids, and antibiotics. Glucocorticosteroids are potent anti-inflammatory drugs that are commonly prescribed in CRSwNP. Topical corticosteroid therapy is the first- line treatment of chronic rhinosinusitis and has an effect on polyp size, nasal symptoms, and recurrence rate. Van Zele et al.63 evaluated the effect of oral glucocorticosteroids in a randomized controlled trial and showed a significant but short-term effect on polyp size and nasal symptoms. In view of the quick recurrence of polyps, they stated that treatment with oral steroids is of limited value unless surgery or treatment with intranasal corticosteroids is combined.63
Antibiotics are a treatment option, as a role for microorganisms is proposed. In particular, macrolides and doxycycline are good choices, because they have an anti-inflammatory action next to their antibiotic features.63 Macrolides like erythromycin, clarithromycin, and roxithromycin affect neutrophils and eosinophils to reduce tissue damage by chronic bacterial colonization. The tetracycline antibiotic family has good tissue penetration in airway tissue, has a broad spectrum, and is especially effective against SA. The effect of doxycycline was examined by Van Zele et al.63 and had a significant, albeit more moderate, effect compared to that of oral glucocorticosteroids. The effect of 3-week doxycycline treatment with 100 mg/day was present for 12 weeks, whereas the effect of tapering methylprednisolone treatment over the same period only lasted for 8 weeks.
By the end of the last century, biological antibodies were developed, including anti-IL5, anti-IL4Rα, and anti-IgE. Unlike corticoids, these specific treatments are only effective in a selected population. Nevertheless, it might be an opportunity to predict responsiveness to a certain targeted treatment based on different T-cell patterns and cytokines in serum, in mucosa when surgery is mandatory, or in nasal secretions.
Considering Th2-skewed eosinophilic inflammation with marked local production of functional IgE antibodies, it can be assumed that treatment with anti-IgE is indicated for this endotype. Especially in patients with nasal polyps and comorbid asthma, treatment targeting IgE can be advocated, as high local IgE is frequently present in this subpopulation (Fig. 2). Indeed, this therapy has proven to be successful in CRSwNP and comorbid asthma with a substantial decrease in nasal polyp scores after 16 weeks in the omalizumab group, as compared to baseline.64 Upper and lower airway symptoms significantly improve by application of anti-IgE therapy in both atopic and non-atopic patients with nasal polyps. The independence of atopic status highlights the functionality of the locally produced IgE. This result is in contrast to that of a study conducted by Pinto65 in 2010. In this randomized controlled trial, the effect of omalizumab was determined in patients with chronic sinusitis, as compared to placebo. An improvement of sinus opacification in CT scans and the SNOT-20 questionnaire was found, but the difference was not significant.65 However, the population also included patients with CRSsNP, which may partially explain the limited benefit from omalizumab; furthermore, the patients were using intranasal corticosteroids, which were not tried to reduce. From both studies, we can conclude that patients have to be selected carefully when considering a treatment or a study with a targeted treatment.
IL4 is, next to IL13, an important component in airway inflammation, as the IL4/IL13/STAT6 pathway is crucial in Th2-mediated inflammation. Several IL4-antagonizing approaches have been proposed, including the soluble recombinant human IL4 receptor, IL4 mutein, humanized anti-IL4 monoclonal antibody, and human anti-IL4 receptor α monoclonal antibody. By targeting the α subunit of the IL4 receptor, both IL4 and IL13 are blocked; this could be more effective than targeting either one alone.66 Dupilumab is a fully human monoclonal antibody targeting the IL4 receptor α subunit. A randomized controlled trial has investigated the efficacy and safety of dupilumab in patients with persistent, moderate-to-severe asthma and a blood eosinophil count of at least 300 cells per microliter or a sputum eosinophil level of at least 3%.67 In view of the good clinical result in asthma, it would be interesting to investigate the efficacy in eosinophilic CRSwNP.
As mentioned before, TSLP is an epithelially derived cytokine that is important in promoting Th2-type inflammation. An increased expression of messenger RNA for TSLP and an increased number of TSLP-positive cells are demonstrated in nasal polyps. Moreover, there is a clear correlation between the number of TSLP-positive cells and the IgE level.68 It would be interesting to assess the effect of TSLP inhibition on the IgE level in CRSwNP. AMG 157 is a human antibody targeting TSLP,69 and thus theoretically this antibody could prevent need to same in wole text IgE formation upstream. Similarly, next to TSLP, IL33, and IL25 represent a link between the epithelium and the Th2-dominated adaptive immunity in CRSwNP (Fig. 1). The IL33/ST2 pathway be a therapeutic target, and blockade by means of anti-IL33 or soluble ST2 could reduce inflammation in nasal polyps. To our knowledge, however, the impact of such blockade on local IgE in CRSwNP has not yet been investigated.
In many sinonasal diseases, IgE is crucial in the pathogenesis. Systemic markers to investigate inflammation in upper airway disease are sometimes not representative for local inflammation. IgE that is locally produced in the target organ could have diagnostic and therapeutic importance in AR and CRSwNP.
Figures and Tables
Fig. 1
Pathways resulting in local IgE production in CRSwNP. In the first part, dendritic cells of the skin and mucosa process aeroallergens deposited on the mucosa, and subsequently they present antigens to T cells. T helper 2 cells release their mediators upon recognition of antigens presented by antigen-presenting cells. The Th2 cytokines IL4, IL13, and CD40L induce selective somatic recombination of immunoglobulin heavy chain regions in B cells before maturation into IgE-producing plasma cells. IL5 stimulates eosinophil growth and differentiation. Alternatively, IgE is produced by stimulating innate lymphoid cells to release IL4, IL5, and IL13.
ACKNOWLEDGMENTS
This study was supported by research grants provided by Ghent University and the Flemish Scientific Research Board (FWO). Els De Schryver receives funds from Special Research fund (BOF, B/11005/02). Philippe Gevaert receives a grant as a senior clinical investigator from the Flemish Scientific Research Board (FWO). This work was also supported by grants to Claus Bachert from the Flemish Scientific Research Board (FWO, Nr. A12/5-HB-KH3 and G.0436.04), and by the Interuniversity Attraction Poles Programs (IUAP) -Belgian state -Belgian Science Policy P6/35 and P7/30, and the Global Allergy and Asthma European Network (GA2LEN).
References
1. Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013; 68:55–63.
2. Durham SR, Gould HJ, Thienes CP, Jacobson MR, Masuyama K, Rak S, et al. Expression of epsilon germ-line gene transcripts and mRNA for the epsilon heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur J Immunol. 1997; 27:2899–2906.
3. Cameron L, Gounni AS, Frenkiel S, Lavigne F, Vercelli D, Hamid Q. S epsilon S mu and S epsilon S gamma switch circles in human nasal mucosa following ex vivo allergen challenge: evidence for direct as well as sequential class switch recombination. J Immunol. 2003; 171:3816–3822.
4. Takhar P, Smurthwaite L, Coker HA, Fear DJ, Banfield GK, Carr VA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005; 174:5024–5032.
5. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289.
6. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000; 102:553–563.
7. Pène J, Rousset F, Brière F, Chrétien I, Bonnefoy JY, Spits H, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988; 85:6880–6884.
8. Luger EO, Fokuhl V, Wegmann M, Abram M, Tillack K, Achatz G, et al. Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol. 2009; 124:819–826.e4.
9. Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF Jr, Curotto de Lafaille MA, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007; 26:191–203.
10. Mølgaard E, Thomsen SF, Lund T, Pedersen L, Nolte H, Backer V. Differences between allergic and nonallergic rhinitis in a large sample of adolescents and adults. Allergy. 2007; 62:1033–1037.
11. KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000; 15:491–497.
12. Eckl-Dorna J, Pree I, Reisinger J, Marth K, Chen KW, Vrtala S, et al. The majority of allergen-specific IgE in the blood of allergic patients does not originate from blood-derived B cells or plasma cells. Clin Exp Allergy. 2012; 42:1347–1355.
13. Takhar P, Corrigan CJ, Smurthwaite L, O'Connor BJ, Durham SR, Lee TH, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007; 119:213–218.
14. Rondón C, Doña I, López S, Campo P, Romero JJ, Torres MJ, et al. Seasonal idiopathic rhinitis with local inflammatory response and specific IgE in absence of systemic response. Allergy. 2008; 63:1352–1358.
15. Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997; 99:1492–1499.
16. Rondón C, Romero JJ, López S, Antúnez C, Martín-Casañez E, Torres MJ, et al. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol. 2007; 119:899–905.
17. KleinJan A, Godthelp T, van Toornenenbergen AW, Fokkens WJ. Allergen binding to specific IgE in the nasal mucosa of allergic patients. J Allergy Clin Immunol. 1997; 99:515–521.
18. Sensi LG, Piacentini GL, Nobile E, Ghebregzabher M, Brunori R, Zanolla L, et al. Changes in nasal specific IgE to mites after periods of allergen exposure-avoidance: a comparison with serum levels. Clin Exp Allergy. 1994; 24:377–382.
19. Yoshida T, Usui A, Kusumi T, Inafuku S, Sugiyama T, Koide N, et al. A quantitative analysis of cedar pollen-specific immunoglobulins in nasal lavage supported the local production of specific IgE, not of specific IgG. Microbiol Immunol. 2005; 49:529–534.
20. Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975; 2:148–150.
21. Smurthwaite L, Walker SN, Wilson DR, Birch DS, Merrett TG, Durham SR, et al. Persistent IgE synthesis in the nasal mucosa of hay fever patients. Eur J Immunol. 2001; 31:3422–3431.
22. Cameron LA, Durham SR, Jacobson MR, Masuyama K, Juliusson S, Gould HJ, et al. Expression of IL-4, Cepsilon RNA, and Iepsilon RNA in the nasal mucosa of patients with seasonal rhinitis: effect of topical corticosteroids. J Allergy Clin Immunol. 1998; 101:330–336.
23. Cameron L, Hamid Q, Wright E, Nakamura Y, Christodoulopoulos P, Muro S, et al. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol. 2000; 106:46–52.
24. Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol. 2003; 171:5602–5610.
25. Snow RE, Chapman CJ, Frew AJ, Holgate ST, Stevenson FK. Pattern of usage and somatic hypermutation in the V(H)5 gene segments of a patient with asthma: implications for IgE. Eur J Immunol. 1997; 27:162–170.
26. Coker HA, Harries HE, Banfield GK, Carr VA, Durham SR, Chevretton E, et al. Biased use of VH5 IgE-positive B cells in the nasal mucosa in allergic rhinitis. J Allergy Clin Immunol. 2005; 116:445–452.
27. Gould HJ, Takhar P, Harries HE, Durham SR, Corrigan CJ. Germinal-centre reactions in allergic inflammation. Trends Immunol. 2006; 27:446–452.
28. Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005; 60:71–79.
29. Ying S, Humbert M, Meng Q, Pfister R, Menz G, Gould HJ, et al. Local expression of epsilon germline gene transcripts and RNA for the epsilon heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. 2001; 107:686–692.
30. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008; 8:205–217.
31. Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013; 132:789–801.
32. Cameron L, Christodoulopoulos P, Lavigne F, Nakamura Y, Eidelman D, McEuen A, et al. Evidence for local eosinophil differentiation within allergic nasal mucosa: inhibition with soluble IL-5 receptor. J Immunol. 2000; 164:1538–1545.
33. Powe DG, Groot Kormelink T, Sisson M, Blokhuis BJ, Kramer MF, Jones NS, et al. Evidence for the involvement of free light chain immunoglobulins in allergic and nonallergic rhinitis. J Allergy Clin Immunol. 2010; 125:139–145.e1-3.
34. Incorvaia C, Fuiano N, Canonica GW. Seeking allergy when it hides: which are the best fitting tests? World Allergy Organ J. 2013; 6:11.
35. Groot Kormelink T, Thio M, Blokhuis BR, Nijkamp FP, Redegeld FA. Atopic and non-atopic allergic disorders: current insights into the possible involvement of free immunoglobulin light chains. Clin Exp Allergy. 2009; 39:33–42.
36. Rondón C, Fernández J, López S, Campo P, Doña I, Torres MJ, et al. Nasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J Allergy Clin Immunol. 2009; 124:1005–1011.e1.
37. Bachert C, Wahl R, Bousquet J, Maasch HJ, Ganzer U. Determination of IgE-specificities in nasal secretions and sera of allergic subjects by crossed radio-immunoelectrophoresis. Clin Exp Allergy. 1990; 20:305–309.
38. Chan MA, Gigliotti NM, Dotson AL, Rosenwasser LJ. Omalizumab may decrease IgE synthesis by targeting membrane IgE+ human B cells. Clin Transl Allergy. 2013; 3:29.
39. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005; 115:459–465.
40. Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003; 91:182–188.
41. Sullivan SD, Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008; 63:670–684.
42. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; 126:962–968. 968.e1–968.e6.
43. Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006; 118:1133–1141.
44. Matsuwaki Y, Uno K, Okushi T, Otori N, Moriyama H. Total and antigen- (fungi, mites and staphylococcal enterotoxins) specific IgEs in nasal polyps is related to local eosinophilic inflammation. Int Arch Allergy Immunol. 2013; 161:Suppl 2. 147–153.
45. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968.
46. Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001; 107:607–614.
47. Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011; 66:141–148.
48. Kang JW, Nahm DH, Suh KS, Kim HY, Park HS. Local production of specific IgE antibody to house dust mite in nasal polyp tissues. J Asthma Allergy Clin Immunol. 1998; 18:426–433.
49. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464:1367–1370.
50. Groot Kormelink T, Calus L, De Ruyck N, Holtappels G, Bachert C, Redegeld FA, et al. Local free light chain expression is increased in chronic rhinosinusitis with nasal polyps. Allergy. 2012; 67:1165–1172.
51. Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007; 37:1840–1847.
52. Hupin C, Rombaux P, Bowen H, Gould H, Lecocq M, Pilette C. Downregulation of polymeric immunoglobulin receptor and secretory IgA antibodies in eosinophilic upper airway diseases. Allergy. 2013; 68:1589–1597.
53. Mechtcheriakova D, Sobanov Y, Holtappels G, Bajna E, Svoboda M, Jaritz M, et al. Activation-induced cytidine deaminase (AID)-associated multigene signature to assess impact of AID in etiology of diseases with inflammatory component. PLoS One. 2011; 6:e25611.
54. Bachert C, Gevaert P, Zhang N, van Zele T, Perez-Novo C. Role of staphylococcal superantigens in airway disease. In : Marone G, editor. Superantigens and superallergens. New York (NY): Karger;2007. p. 214–236.
55. Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004; 114:981–983.
56. Bachert C, Gevaert P, Howarth P, Holtappels G, van Cauwenberge P, Johansson SG. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol. 2003; 111:1131–1132.
57. Krysko O, Holtappels G, Zhang N, Kubica M, Deswarte K, Derycke L, et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy. 2011; 66:396–403.
58. Suh YJ, Yoon SH, Sampson AP, Kim HJ, Kim SH, Nahm DH, et al. Specific immunoglobulin E for staphylococcal enterotoxins in nasal polyps from patients with aspirin-intolerant asthma. Clin Exp Allergy. 2004; 34:1270–1275.
59. Suh KS, Park HS, Nahm DH, Kim YK, Lee YM, Park K. Role of IgG, IgA, and IgE antibodies in nasal polyp tissue: their relationships with eosinophilic infiltration and degranulation. J Korean Med Sci. 2002; 17:375–380.
60. Sheahan P, Ahn CN, Harvey RJ, Wise SK, Mulligan RM, Lathers DM, et al. Local IgE production in nonatopic nasal polyposis. J Otolaryngol Head Neck Surg. 2010; 39:45–51.
61. Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. 2014; 44:553–562.
62. Calus L, Devuyst L, De Ruyck N, Van Zele T, Bachert C, Gevaert P. Nasal allergen provocation test in nasal polyposis with and without allergy. Clin Transl Allergy. 2013; 3:O14.
63. Van Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010; 125:1069–1076.e4.
64. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; 131:110–116.e1.
65. Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010; 48:318–324.
66. Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010; 181:788–796.
67. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–2466.
68. Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011; 3:186–193.
69. Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014; 370:2102–2110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download