Abstract
Sorafenib is an oral multikinase inhibitor with clinical activity against hepatocellular carcinoma (HCC) and renal cell carcinoma. Administration of sorafenib carries a variety of adverse cutaneous reactions. Common adverse effects induced by sorafenib include hand-foot skin reactions, facial erythema, splinter subungual hemorrhage, and alopecia. Although erythema multiforme (EM) related to sorafenib has been reported, delayed-type cutaneous hypersensitivity reactions are rare in patients treated with sorafenib and there has been no case of Stevens-Johnson syndrome (SJS) reported so far. We recently experienced 3 cases of delayed-type cutaneous hypersensitivity related to administration of sorafenib. The first case was a 47-year female had targetoid erythematous rashes on her arms 12 days after starting sorafenib for HCC. The rashes spread from the arms to the trunk rapidly except for the hands and feet, and erosive lesions developed in the oral mucosa and lips. She was diagnosed as SJS. The second case was an 81-year-old male had maculopapular eruptions with multiple targetoid lesions on the trunk, arms, and legs 10 days after starting sorafenib for his HCC. There was no evidence of mucosal involvement. He was diagnosed with EM. The last one was a 20-year-old female developed generalized maculopapular eruptions in the whole body 10 days after starting sorafenib for the treatment of HCC. All 3 patients completely recovered after discontinuation of sorafenib.
Sorafenib (Nexavar®, Bay 43-9006) is an oral multitargeted kinase inhibitor which selectively inhibits tyrosine protein kinases, such as the vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, the platelet-derived growth factor receptor-β, and Raf kinase. Sorafenib was approved as an effective agent for treating hepatocellular carcinoma (HCC) and metastatic renal cell carcinoma by the Food and Drug Administration in 2005.
Dermatologic adverse events occur in most of the patients treated with sorafenib.1 Most frequently occurring cutaneous effects are hand-foot skin reaction (HFSR), uncharacterized skin eruption, subungual splinter hemorrhage, alopecia, pruritus, dry skin, and flushing.2,3 However, typical delayed-type cutaneous hypersensitivity reactions are not common. There have been only a few reports of sorafenib-induced erythema multiforme (EM) so far.4,5,6,7 However, other severe forms of delayed-type hypersensitivity reactions have not yet been reported. We herein report 3 cases of sorafenib-induced delayed-type cutaneous adverse reactions with their human leukocyte antigen (HLA) information.
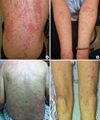
A 47-year-old female diagnosed as having HCC with adrenal metastases was treated with 400 mg of sorafenib twice a day. On the 12th day of therapy, erythematous rashes with targetoid lesions developed on her both arms and ulcers occurred in the oral mucous membrane and lips. In a complete blood count test, hemoglobin was 15.7 g/dL, total leukocytes 4,240/mm3 (eosinophil: 8.5%), and platelets 114,000/mm3. The following day, rashes rapidly spread over the whole body and became confluent patches or plaques on her chest, upper abdomen, and both upper and lower extremities without involving of palms and soles (Figure. A, B). A diagnosis of Stevens-Johnson syndrome (SJS) was made, and all drugs were withdrawn. Oral prednisolone 15 mg/day started, and her skin lesions disappeared within 1 week. One month after the skin rash completely cleared up, sorafenib was readministered with caution at the charge physician's request but discontinued on the second day because of pruritic erythematous eruptions and high fever. HLA class I loci were genotyped, and her HLA class I alleles were as follows: B*46:01/B*13:01, Cw*02:07/Cw*02:07, and A* 24:02/A*33:03 (Table).
An 81-year-old male diagnosed as having HCC with multiple lymph node metastases was started treatment with 400 mg of sorafenib twice a day and experienced multiple generalized targetoid bullous lesions on the 10th day. Between days 10 and 12, the skin lesions spread over his trunk and extremities (Figure. C, D). The hands and feet, especially the palms and soles, were free of lesion. Fever rose up to 38.4℃. Unlike case 1, he did not show any mucosal involvement. He was diagnosed as having EM. Other than sorafenib, no drugs were newly administered within 1 month before EM developed. After discontinuation of sorafenib, the cutaneous lesions disappeared without steroid treatment. His HLA class I alleles were as follows: B*46:01/B*15:01, Cw*04:01/Cw*01:02, and A* 26:01/A*02:01 (Table).
A 20-year-old female diagnosed as having HCC with ovarian metastases received 400 mg of sorafenib twice a day. She experienced generalized maculopapular eruptions (MPE) on the whole body on the 10th day of sorafenib administration. Sorafenib administration was discontinued, and the patient was treated with oral prednisolone 30 mg/day for 2 days. MPE completely disappeared week after discontinuation of sorafenib. Her HLA class I alleles were as follows: B*35:01/B*07:05, Cw*04:01/Cw15:02, and A* 24:02/A*31:01 (Table).
Cutaneous adverse reactions are frequently detected in up to 70%-90% of patients treated with sorafenib, and most of the dermatologic problems are type A toxic reactions.2 HFSR is the most commonly occurring toxic adverse reaction, shown in 6%-72% of patients.2,8 These toxicities are often correlated with the efficacy of treatment,9 usually reversible with the use of topical therapies, and not life-threatening. Therefore, permanent discontinuation is not required. Other than these dermatologic toxicities, sorafenib rarely induces delayed-type cutaneous hypersensitivity reactions, such as true MPE or EM. Furthermore, no cases of sorafenib-induced severe cutaneous adverse reactions (SCARs), such as SJS, toxic epidermal necrolysis, or drug rash with eosinophilia and systemic symptoms (DRESS), have been reported yet.
We experienced 3 cases of delayed-type cutaneous adverse reactions. In case 1, there were blisters and ulcerations on the oral mucosa and lips with disseminated erythematous edema saving the palms and soles, and it was clinically compatible with a diagnosis of SJS. To our knowledge, this is the first reported case of sorafenib-induced SJS. In case 2, thumb-sized erythema developed on the trunk and limbs, and in case 3 generalized morbilliform eruptions initially appeared on the trunk and spread to the whole body. We diagnosed cases 2 and 3 as EM and MPE, respectively.
Current practice in diagnosing adverse drug reactions mainly based on clinical features, patient's medication history, and clinical course after discontinuation of the culprit drug has an intrinsic limitation in that the pathophysiology cannot be proved. However, we assessed that the clinical features that our patients presented were caused by delayed-type hypersensitivity reactions considering the following. First, there was a time interval of approximately 11.3 days in all of the 3 patients between the time of sorafenib administration and the onset of the symptom. It suggests that there have been immunologic responses requiring time lag to be sensitized and to gain immunologic memory.7 Second, the features of skin lesions support the involvement of type IV hypersensitivity reaction. Although our patients did not undergo biopsy, previous studies of sorafenib-induced EM have revealed CD8+ lymphocytes infiltration into the epidermis and necrotic keratinocytes.6,10 One of the studies also has shown positive patch test reactions to sorafenib in all EM patients.10 Though MPE is a milder and more common form of hypersensitive reaction to sorafenib, there is some evidence that CD8+ lymphocytes infiltrate into the lesion of MPE along with CD4+ lymphocytes.11 These findings, time interval required, and involvement of CD8+ lymphocytes support that MPE, EM, and SJS can be developed by a delayed-type hypersensitivity to sorafenib, although they are not disorders of the same spectrum.
EM can be caused by a delayed-type hypersensitivity reaction to certain drugs and by some infectious agents presenting with characteristic iris lesions. Postmarketing surveillance of sorafenib reported that the incidences of sorefenib-induced EM were only 0.1% to less than 1%. A Japanese phase 2 study and a phase 3 target study reported that EM occurred in 1.5% and 2.4% of patients, respectively.12,13 However, according to a recent Japanese study conducted prospectively focusing on skin adverse reactions, it was reported that EM occurred in 9 (25%) out of 36 patients.10 It seems that sorafenib-associated EM might be more common than expected. We need to study more cases and investigate the incidence of EM in Koreans because patterns of HLA distribution and associations with drug hypersensitivity are relatively similar in Korean and Japanese populations.
Drug-induced SCARs are usually classified as a type B adverse drug reaction associated with an immunological reaction.14 Because of their serious morbidity and mortality, there has been a sustained effort to find markers that can predict individual susceptibility to these conditions. Some HLAs are known as genetic markers for the development of SCAR by certain drugs, such as allopurinol, carbamazepine, and abacavir.15,16 For example, the allele HLA-B*58:01 was found in almost 100% of patients who developed DRESS in the Asian population.16 For sorafenib hypersensitivity, there was no genetic study reported up to now. We assessed the HLA alleles of our patients and found that cases 1 (SJS) and 2 (EM) share the allele HLA-B*46:01 which is relatively uncommon in the Korean general population (8.87%).17 Considering SJS and EM show more closely related pathologies, such as bullous formation and CD8+ infiltration in affected area, the allele HLA-B*46:01 seems to indicate relevance to these features. Case 3 with MPE also shares the allele HLA allele A*24:02 with case 1 and Cw*04:01 with case 2, but these alleles are more commonly found in the Korean population (38.76% and 12.78%, respectively) than HLA-B*46:01.
To increase the overall sensitivity of diagnosis, it is preferable to supplement additional studies in vivo and in vitro. However, due to the condition and grave prognosis of the underlying malignancy of the presented patients, it was hardly possible to proceed to perform any diagnostic test. To confirm the diagnosis and clarify their genetic predisposition, further studies with a larger number of well characterized patients affected by sorafenib are warranted.
It is not easy to differentiate delayed-type hypersensitivity reactions from type A drug reactions but misdiagnosis can result in serious morbidity and mortality because a more severe and fatal reaction may occur by readministration of the culprit drug in case of delayed-type hypersensitivity reactions. Therefore, physicians should keep in mind that sorafenib can trigger severe delayed-type hypersensitivity, and when it is suspected, readministration should be avoided.
We reported 3 cases of delayed-type cutaneous reactions induced by sorafenib, including the first case of sorafenib-induced SJS. Physicians should recognize that severe delayed-type hypersensitivity reactions can develop after sorafenib administration and that these reactions are contraindications into the readministration of sorafenib.
Figures and Tables
Table
Phenotype frequencies of the HLA-B*46:01, Cw*04:01, and A*24:02 in Korean general population and this case series

| HLA | Phenotype frequency in Korean general population (%) | Among case patients in the present study |
|---|---|---|
| B*46:01 | 8.87 | Case 1, case 2 |
| Cw*04:01 | 12.78 | Case 2, case 3 |
| A*24:02 | 38.76 | Case 1, case 3 |
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Korea (Grant No. 03-PJ10-PG13-GD01-0002) and the Ministry of Food and Drug Safety to operation of the regional pharmacovigilance center in 2014.
References
1. Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008; 144:886–892.
2. Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008; 158:592–596.
3. Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008; 13:1001–1011.
4. MacGregor JL, Silvers DN, Grossman ME, Sherman WH. Sorafenib-induced erythema multiforme. J Am Acad Dermatol. 2007; 56:527–528.
5. Bilaç C, Müezzinoğlu T, Ermertcan AT, Kayhan TC, Temeltaş G, Oztürkcan S, et al. Sorafenib-induced erythema multiforme in metastatic renal cell carcinoma. Cutan Ocul Toxicol. 2009; 28:90–92.
6. Kodaira M, Takahashi S, Takeuchi K, Yuasa T, Saotome T, Yonese J, et al. Sorafenib-induced erythema multiforme for metastatic renal cell carcinoma. Ann Oncol. 2010; 21:1563–1565.
7. Namba M, Tsunemi Y, Kawashima M. Sorafenib-induced erythema multiforme: three cases. Eur J Dermatol. 2011; 21:1015–1016.
8. Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006; 24:2505–2512.
9. Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010; 15:85–92.
10. Ikeda M, Fujita T, Mii S, Tanabe K, Tabata K, Matsumoto K, et al. Erythema multiforme induced by sorafenib for metastatic renal cell carcinoma. Jpn J Clin Oncol. 2012; 42:820–824.
11. Yawalkar N, Egli F, Hari Y, Nievergelt H, Braathen LR, Pichler WJ. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000; 30:847–855.
12. Akaza H, Tsukamoto T, Murai M, Nakajima K, Naito S. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol. 2007; 37:755–762.
13. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007; 356:125–134.
14. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994; 331:1272–1285.
15. Pirmohamed M. Genetic factors in the predisposition to drug-induced hypersensitivity reactions. AAPS J. 2006; 8:E20–E26.
16. Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011; 21:303–307.
17. Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005; 65:437–447.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download