Abstract
Allergic reactions to fungi were described 300 years ago, but the importance of allergy to fungi has been underestimated for a long time. Allergens from fungi mainly cause respiratory and skin symptoms in sensitized patients. In this review, we will focus on fungi and fungal allergens involved in respiratory forms of allergy, such as allergic rhinitis and asthma. Fungi can act as indoor and outdoor respiratory allergen sources, and depending on climate conditions, the rates of sensitization in individuals attending allergy clinics range from 5% to 20%. Due to the poor quality of natural fungal allergen extracts, diagnosis of fungal allergy is hampered, and allergen-specific immunotherapy is rarely given. Several factors are responsible for the poor quality of natural fungal extracts, among which the influence of culture conditions on allergen contents. However, molecular cloning techniques have allowed us to isolate DNAs coding for fungal allergens and to produce a continuously growing panel of recombinant allergens for the diagnosis of fungal allergy. Moreover, technologies are now available for the preparation of recombinant and synthetic fungal allergen derivatives which can be used to develop safe vaccines for the treatment of fungal allergy.
Already at the beginning of the 18th century, long before the term "allergy" was coined by Clemens von Pirquet, fungal exposure was recognized as a potential cause of adverse respiratory symptoms.1 In 1726, Sir Floyer reported for the first time a severe asthma attack in a patient who had visited a wine cellar where must was fermenting.2 More than 100 years later, Blackley described bronchial catarrh and chest tightness after inhalation of Penicillium glaucum spores.3 In 1924, Storm van Leeuwen suggested that inhaled fungal spores could cause asthma.
Although the first observation of fungal allergy dates back 300 years, the association between exposure to fungi and the occurrence of allergic symptoms has been discussed controversially for a long time, and indeed fungi are still a neglected allergen source.4,5,6 Nowadays, data from several epidemiological studies provide evidence for the important role of fungi in respiratory disease in the indoor as well as in the outdoor environment. Fungal sensitization is not only found more often in patients with asthma but also may represent a risk factor for development of asthma.7,8 Moldy odor, home dampness and visible mould growth have been associated with the development of asthma and the severity of respiratory symptoms in children.9 Indoor exposure to fungi was further found to be a risk factor for the prevalence of coughing, increased peak expiratory flow variability, and asthma.10,11,12,13,14 A correlation was found between spore levels and the occurrence of hospital delivery and asthma treatment, but also with asthma-related death. In Chicago, for example, death from asthma was about twice as common on days with a high total mold spore count as on days with lower spore counts.15 In rural areas, respiratory symptoms have been linked to increased amounts of spores during crop ripening, harvest, and storage.16,17,18 Furthermore, thunderstorm-related asthma was found to be associated with increased spore concentrations of fungal species from Ascomycota and Basidiomycota.19
The importance of molds to induce IgE-mediated reactions was demonstrated by IgE binding experiments, the ability to induce histamine release in basophils from sensitized patients and by skin testing.20,21,22,23 Bronchial and nasal challenge tests with extracts of fungal spores or mycel were able to induce rhinitis, asthma,23,24 and late phase reactions including eosinophilic infiltration.25,26 The fact that extracts of spores and mycel are able to efficiently induce allergic symptoms provided evidence that relevant allergens are present in both spores and mycel.22,23 For Alt a 1, the major Alternaria alternata allergen, the presence in spores and mycel has further been demonstrated by immunogold electron microscopy.27,28,29 Interestingly, fungi also activate the innate immune system and may enhance inflammation caused by unrelated allergens (e.g., grass pollen).30,31,32
A prerequisite for the development of allergic sensitization is that individuals are exposed to the allergen source in clinically relevant amounts. Fungal spores are an ever present component of the atmosphere,18 constituting the largest proportion of aerobiological particles in our environment33 which even exceeds the concentration of pollen grains.17 Due to the large spectrum of mold species and the difficulty in identifying them our knowledge of outdoor atmospheric mold spores and their relevance in allergic disease is still incomplete. A recent study identified a set of 40 potentially allergy-inducing genera in air samples collected in Korea.34
Using DNA analysis differences in the relative abundance and seasonal cycles of various groups of fungi in coarse and fine particulate matter were found, with more plant pathogens in the coarse fraction and more human pathogens and allergens in the respirable fine particle fraction (<3 micron).35
The number of fungal spores in the atmosphere underlies seasonal as well as interdural variations. Especially climatic factors (temperature, rainfall, relative humidity, and wind) and circadian patterns (darkness and sunlight) influence the spectrum of fungal species and their concentrations in the environment.16,33,36,37 The number of spores in the air rises in particular in late summer and early autumn, when nutritional sources are available, while they diminish in winter (Fig. 1).16,37 Considering daily fluctuations, the highest spore counts can be found in the afternoon and early evening.16
In general, exposure to fungi occurs via inhalation, skin contact, or ingestion.38 The inhalative route is with regard to respiratory symptoms the most important one. Fungal spores show a broad spectrum of different shapes and sizes, ranging from less than 2 to 250 µm. A substantial proportion of spores are small enough to penetrate into the lower airways. The threshold level necessary to elicit allergic symptoms in sensitized patients is not known and varies between different species. Spore concentrations of Alternaria equal or greater than 100 spores/m3 are believed to evoke allergic symptoms,39 whereas the reference value for Cladosporium is estimated to be 3,000 spores/m3.18,33 However, for 2 reasons the determination of spore concentrations is not a perfect method to quantify the exposure of a sensitized individual. Using a Halogen Immunoassay (HIA), it was shown that fungal fragments and submicron particles of intra- and extracellular fungal structures contain detectable amounts of allergens and may therefore function as aeroallergen sources.40 These particles are present in considerable larger quantities than spores, and respiratory deposition models indicate for some fungal species a much higher burden caused by fragments than by spores.41 Second, there is evidence that airborne allergen levels do not always correspond to spore or particle counts.42,43 For example, it has been shown that depending on the developmental status, spores from Alternaria alternata release different amounts of the major allergen Alt a 1.44
Fungi are ubiquitous and, as a consequence of this, sensitization to fungi can be found throughout the world (Fig. 2). The exact prevalence of mold sensitization is not known but is estimated to range from 3% to 10% in the general population. Likewise, the prevalence of sensitization in atopic patients varies depending on many factors as it was exemplified in several large studies.45,46,47,48,49 A multicenter study in 7 European countries investigated the prevalence of Alternaria and Cladosporium sensitization in 877 children and adults (5-60 years) with rhinits and/or asthma. From these, approximately 9.5% were skin prick positive to at least one or both fungal species. The highest prevalence was found in Spain (20%), the lowest in Portugal (3%).50 In another very recent multinational study (EPAAC, Early Prevention of Asthma in Atopic Children) conducted in 10 European countries, Australia, and South Africa, specific IgE antibodies to food and aeroallergens were determined in 2,184 infants (mean age: 17.6 months) with atopic eczema. The overall sensitization rate to Alternaria in this population was 3.7%, but variations were observed between the different countries. Children from Australia showed the highest sensitization rate (7%), whereas in Belgium no sensitization to Alternaria was found.51 The third National Health and Nutrition Examination Survey in the USA revealed that approximately 12.9% of the population (6-59 years) showed positive skin prick results to Alternaria alternata.52 A survey performed by Global Allergy and Asthma European Network in 16 European countries showed general sensitization rates of 11.9% for Alternaria alternata and 5.8% for Cladosporium herbarum with the highest prevalence in the UK, Ireland, and Northern Europe.45 A study performed with atopic patients in a tropic environment demonstrated the importance of sensitization to basidiomycete Ganoderma applanatum with the prevalence of 30%.53 In those studies, populations from different countries have been investigated that varied in age and clinical manifestations of allergy. Thus, it seems that demographic and epidemiological factors contribute to varying prevalence data. Based on studies that consider the age of patients in the evaluation of prevalence data, it was assumed that sensitization to fungi is an age-dependent process with a higher prevalence in children.11,51,52,53,54,55,56,57 It was further shown for allergy to the species Alternaria that the course of the disease differed markedly from allergies to other common aeroallergens.58 Alternaria-specific IgE antibodies increase significantly in early childhood until they reach a maximum level and then seem to decline again with increasing age.11,55,56,57,58 In contrast to this, specific IgE antibodies to house dust mite increase with age and remain constantly high during childhood.11 It is interesting that monosensitization to fungi is quite rare and that specific IgE to fungi are often associated with the presence of specific IgE to other aeroallergens.54,59 So far, the reason for this polysensitization in connection with fungal sensitization is not known. A study in 6,840 Italian children pointed also at the influence of genetic factors, as 82.9% of mold-sensitized children had a positive family history of mold allergy.59
The varying prevalences of mold sensitization may also be ascribed to methodological limitations. Current methods for identification of mold species rely especially on morphological characteristics of spores, so they are time-consuming and require the skills of a trained person. As only a small number of mold species have been identified, sensitization to unknown fungi remains elusive. Furthermore, one has to be aware of the fact that results of skin tests and in vitro measurements of specific IgE antibodies are often not directly comparable.57,60,61 Finally, fungal allergen extracts used for diagnostic testing are of poor quality and show high variability regarding allergen contents.62 Although at present the prevalence of sensitization to fungi can only be estimated, many studies identify fungi as an important cause of respiratory allergy.
It has been estimated that around 1 to 1.5 million fungal species exist worldwide, but so far only 80,000 have been described. From these, 112 genera are thought to be a source of allergens. The 4 genera most commonly associated with the development of allergy are Alternaria,54 Cladosporium, Penicillium, and Aspergillus. These genera belong to the phyllum Ascomycota, but allergens have also been described from Basidiomycota and Zygomycota. Overall, 107 allergens from 28 fungal genera have been approved by the International Allergen Nomenclature Sub-committee (http://www.allergen.org/, Fig. 3), but many fungal proteins have been shown to be IgE-reactive. Bowyer et al.38 estimated that the most thoroughly studied allergenic fungi have up to 20 well characterized allergens and further 27 to 60 other less well characterized IgE binding proteins. Recently, it was shown that IgE sensitization to fungal species reflected well their phylogenetic relationship since IgE reactivity correlated better in closely related molds compared to phylogenetically distant molds.63 Supplementary Table 1 summarizes known fungal allergens and gives an overview on their biological function, their prevalence of IgE recognition, and their cross-reactivity.
Although molds produce a great variety of IgE-binding molecules, not all of them are equally important. The first factor that determines the importance of an allergen is the prevalence of recognition among patients sensitized to the allergen source. Based on this, an allergen can be classified as major (>50%) or minor (<50%) allergen. However, it is known that IgE reactivity of an allergen does not necessarily reflect its capacity to induce allergic symptoms. Determination of the IgE-binding capacity is therefore not sufficient for assessing the clinical relevance of an allergen. It is rather the capability to induce strong IgE-mediated and T cell-mediated reactions that define the clinical relevance. The ability of an allergen to induce IgE-mediated reactions can be assessed by in vitro (basophil histamine release) and in vivo tests (skin and provocation tests). Additionally, T-cell proliferation assays (in vitro) and the atopy patch test (in vivo) allow inferences on T cell-mediated processes.
The clinical relevance is further dictated by the frequency of the allergen's occurrence and the amount of allergen liberated by a mold species. Molds and fragments thereof are not only a constant component of our environment, but under certain conditions that favor their growth (i.e. moisture, poor ventilation, etc.) exposure may be exceptionally high. Antibody probes against mold allergens are useful tools to determine the presence of a certain fungus and to measure the personal allergen exposure.
Knowledge about the sequence and structure of an allergen forms the basis for the understanding of its immunological and biological properties and ultimately is essential for the development of new forms of diagnosis and treatment.64 To study these characteristics, the allergen needs to be available with a high degree of purity. First allergen characterizations were carried out on natural allergens isolated from allergen extracts, but complex and tedious purification processes hampered such analysis for many allergen sources. With the introduction of recombinant DNA technology into the field of allergology, an ongoing progress in allergen characterization started more than 25 years ago. Today, many mold allergens have already been cloned (Supplementary Table 1) and are available as recombinant proteins. The amino acid sequence of an allergen determines its physiochemical characteristics and forms the basis of the secondary and tertiary structures. So far, the 3 dimensional structures of some mold allergens have been solved by X-ray crystallography.65,66,67,68,69,70 Computational structure prediction programs in principle allow predictions of the allergen's conformation based on the amino acid sequence. Knowledge of the sequence further facilitates the identification of amino acids involved in IgE binding and T-cell activation.71 Currently increasing numbers of B and T-cell epitopes of allergens from different mould species are determined.72,73
Furthermore, the knowledge about similarities in sequence and conformation also permits the identification of homologous proteins in related and unrelated species, which might have cross-reactive potential. However, one has to be aware of the fact that the degree of sequence identity between an allergen and a homologous protein does not necessarily predict cross-reactivity. Overall, cross-reactivity between proteins can be due to common moieties in the protein sequence or to common carbohydrate structures (CCD, cross-reactive carbohydrate determinant). It can thus occur between proteins from taxonomically related, but also from distantly or non-related sources. Hence, cross-reactivity has been found within 1 fungal phylum, between different fungal phyla and also to non-fungal allergens of other allergen sources74 and even to human proteins. The most prominent examples of cross-reactive fungal allergens are enolases,75,76,77 manganese superoxide dismutases (MnSOD),78 cyclophilins,66,79,80 glutathione-S-transferases,81 thioredoxins,67,82 and transaldolases.83 Beside these, serine proteases, ribosomal proteins, heat shock proteins, and peroxisomal proteins have found to be cross-reactive (see supplementary Table 1). Clinically, cross-reactive molecules are responsible for IgE-mediated reactions to a variety of allergen sources. Cross-reactive molecules with poor allergenic activity (e.g., CCDs) can influence the accuracy of diagnosis since tests based on IgE binding give positive results that do not reflect the clinical importance of the allergens. In addition, the relevance of CCDs in eliciting IgE-mediated reactions is still a matter of controversy and, although many mold species produce glycosylated proteins that bind IgE in vitro, it is not clear whether the carbohydrate moiety contributes to the allergenicity of the molecule. The lack of biological activity might be due to a limited epitope valency of carbohydrates and/or a low affinity of IgE antibodies for the corresponding glycan epitope.84,85 In case of plant carbohydrates, structures that are crucial for IgE antibody binding have been determined: asparagine-linked xylose and fucose residues are mainly responsible for structural similarities which cause cross-reactivity.84,85 In contrast to plant carbohydrates, mold proteins are highly mannosylated, indicating that plant and fungal CCDs are not cross-reactive; however, further research is required to address this issue.
Knowledge about an allergen's sequence can give information regarding the biological and biochemical properties of a protein. Since molds are heterotrophic organisms, they are dependent on organic nutrients provided by other organisms. They digest their food externally, excreting enzymes into the environment.33 It is therefore not surprising that many fungal allergens have enzymatic functions. However, enzymatic activity may also have an influence on the allergenicity of an allergen source. First, proteases of fungal extracts were shown to have Th2-inducing properties86 either by facilitating antigen access through cleavage of tight junction proteins87 or through direct activation of epithelial cells.87,88,89,90,91,92,93 Second, degradation processes can impair the stability of other allergens present in an allergen source.
To summarize, the knowledge regarding the structures, immunological and biological properties of mold allergens is increasing, but there is a need for the systematic evaluation of the prevalence of IgE recognition of individual allergens in population studies and of the allergenic activity of allergens to determine their clinical relevance. Only on the basis of such systematic studies, new forms of diagnosis and therapy can be developed.
The first and most important step toward efficient allergen-specific forms of treatment is the proper diagnosis of mold sensitization and the evaluation of the clinical relevance of the sensitizing allergens. Diagnosis of allergies, as routinely performed today, is a stepwise process, including anamnesis, determination of total and allergen-specific IgE antibodies, skin tests and, if necessary, other provocation tests.94 Anamnesis plays a decisive role in the diagnosis of allergy and usually guides further diagnostic steps, such as serological testing and provocation testing. However, if fungal allergy is not considered, it may be easily overlooked because fungal allergens often co-occur with other frequent indoor (e.g., house dust mites, animal dander) and outdoor allergens (grass and weed pollen). In fact, many of the mold-sensitized patients have also specific IgE antibodies to other inhalant allergen sources, and peak spore levels overlap with grass and weed pollen season, so mold sensitization might be masked by other allergies (Fig. 4).
Allergen-specific IgE antibodies and their clinical relevance are basically determined by serological investigations and skin tests. In general, skin tests are more commonly found to correlate with clinical symptoms of allergy and are regarded as the more sensitive tests, whereas serological investigations are considered more specific.95 A combination of in vivo and in vitro tests is therefore recommended for a reliable diagnosis of mold allergy.96 A recent study on diagnosis of mold allergy compared the sensitivity of an intradermal skin test with a serum assay for specific IgE antibodies (ImmunoCAP test) and showed impressively the gap between in vivo and in vitro results. Seventy-five patients with rhinitis were tested with extracts of Candida, Alternaria, Cladosporium, and Penicillium for their IgE reactivity. The incidence of a positive test determined by skin tests in comparison to the CAP analysis varied fewest for Cladosporium (4-fold) and the most for Alternaria (17.5-fold).61 A multicenter study from Spain which determined the prevalence of Alternaria alternata sensitization revealed that in more than one-third of patients, a positive in vivo result could not be confirmed with the in vitro tests.57
The discrepancy of diagnostic tests can partly be ascribed to the poor quality of mold extracts.95 Besides intrinsic factors, like strain variabilities97,98,99,100,101,102 and the tendency of molds for spontaneous mutation,103 the raw material (spores/mycelium)20,21,22 and manufacturing processes (culturing conditions, extracting procedures)28,97,99,100,104,105 affect the quality of mold allergen extracts. As molds are enzyme-rich organisms, degradation processes should also not be underestimated.97,99,100,102,104 All these factors may have an impact on the presence of certain allergens, protein/carbohydrate content, and allergenicity and antigenicity of mold extracts.28,97,98,99,100,104,105 Martínez et al.101 reported high variabilities in the expression of the major allergen Alt a 1 in 11 Alternaria alternata strains that were cultured under identical conditions. In the same mold species, Sáenz-de-Santamaria et al.102 observed varying enzymatic activities in 14 strains. Portnoy et al.104 revealed that extraction time influences the elution of certain allergens and that a longer extraction interval increases the proportion of carbohydrates. We investigated the influence of mold strains, growth medium, and growth time on the expression of Alternaria alternata allergens. Four Alternaria strains were cultured for 2 or 4 weeks on 3 media that differed in carbohydrate and protein content as well as in the presence of additional nutrients. The immunblot in Fig. 5 displays the enormous impact of the strains, nutritional sources, and growth periods on the prescence of IgE-reactive proteins in the allergen extracts. It also exemplifies the difficulties in defining optimal growth conditions.
Although commercially available allergen solutions have to pass through some company-internal standardization procedures and quality controls, there are until now no generally accepted guidelines for the preparation of allergenic mould extracts. Determination of the protein content and measurement of total IgE activity seem to be unsuitable, as protein concentration might not necessarily be correlated with the allergenicity of the extract, since significant qualitative differences in allergen composition have been described.21,97,98,104 It is therefore not astonishing that considerable differences in potency of mold extracts between manufacturers and even batch-to-batch variations can be found in these so-called "standardized extracts".62,97,106 Vailes et al.106 determined the content of Alt a 1 and Asp f 1 in extracts of Alternaria alternata and Aspergillus fumigatus used for diagnosis in the United States. The extracts were obtained in 2 consecutive years from 8 different manufactures. Whereas Alt a 1 was detectable in all but 1 extract, the amount of Asp f 1 was low or even undetectable in extracts of 4 companies. Furthermore, substantial variations within and between companies were found for Asp f 1. Due to the problems with standardization of mold extracts, only a limited number of them are available for diagnosis. This has certainly contributed to the fact that mold allergy has been underestimated for a long time.
Besides the inconsistency of allergen extracts, the presence of carbohydrates and other cross-reactive components hampers the precise identification of the disease eliciting mold species. Molds are known to contain many glycosylated proteins that might impair diagnostic tests. False positive results due to carbohydrates can be unveiled in in vivo provocation tests, since it is assumed that anti-carbohydrate IgE antibodies lack biological relevance. However, cross-reactive allergens from related and unrelated allergen sources distract from the primary sensitizing source. Especially in case of polysensitized patients, diagnosis based on extracts allows no discrimination between co- and cross-sensitization. The need for an improved component-resolved diagnosis is therefore evident, and it is clear that improvement can only come from the use of recombinant mold allergens.
In fact, allergens from different mold species have been cloned and produced as recombinant IgE-reactive proteins (see supplementary Table 1). For some of them, the ability to induce immediate-type reactions has been demonstrated by basophil histamine release assays and skin prick tests.107,108 Recombinant allergens with similar characteristics as their natural counterpart are suited for in vitro and in vivo diagnosis. The application of species-specific allergens (like Alt a 1, Asp f 1, Cop c 1, or Mala s 1) allows the identification of genuine sensitization to a single mold species. Recombinant mold allergens with known cross-reactivity are additionally useful tools as marker allergens to identify cross-sensitization. In this way, sensitivity as well as specificity of diagnosis can be improved. This concept has been tested in a small group of patients sensitized to Alternaria alternata. A combination of the species-specific major allergen Alt a 1 with the cross-reactive enolase Alt a 6 (formerly Alt a 2) could correctly diagnose sensitization in 7 tested patients.109 Recently, a study including 80 European patients showed that rAlt a 1 can be used to diagnose 98% of patients with allergy to Alternaria alternata and that almost all specific IgE in these patients was directed against Alt a 1.72 This finding suggests that Alt a 1 can be used as a reliable diagnostic marker allergen for genuine sensitisation to Alternaria and could replace the Alternaria extract. However, another study suggested that a recently cloned cross-reactive allergen from Alternaria alternata, namely MnSOD, should be included together with Alt a 1 and Alt a 6 in the molecular array for the diagnosis of allergy to Pleosporaceae since 2 out of 30 patients did not react to Alt a 1 nor to Alt a 6, but they showed reactivity to MnSOD.110 In another study, 2 recombinant proteins from Aspergillus fumigatus, rAsp f 4 and rAsp f 6, were shown to allow the discrimination between allergic bronchopulmonary aspergillosis and Aspergillus sensitization.108 Sensitization to certain mold allergens may also be used to predict the severity of clinical symptoms. Alternaria alternata sensitized patients were reported to be affected more frequently by asthma than non-Alternaria-sensitized patients.111
In summary, mold allergy has long been underestimated and occurs more frequently than expected. Since mold allergic patients are often polysensitized, IgE antibodies to other allergen sources might mask mold allergy. Therefore, the precise determination of the disease-causing allergen source is particularly important for mold allergy and is the basis to correctly prescribe the most appropriate specific forms of treatment.
Treatment of allergic disease is based on pharmacotherapy, allergen avoidance and immunotherapy.112 Whereas pharmaceutical drugs like steroids and antihistamines can solely abate the symptoms of allergic disease, allergen avoidance is in case of outdoor exposure difficult to achieve.113 In case of indoor exposure, avoidance is possible under certain conditions, such as visible mold growth.114 Specific immunotherapy would be an antigen-specific, long-lasting and disease-modifying approach for the treatment of mold allergy. In 1998, the WHO published a position paper with guidelines for safe and effective immunotherapy. These guidelines stressed the point that immunotherapy should only be performed with well-defined vaccines in carefully selected patients.112 In case of mold allergy, these criteria are difficult to fulfill and therefore - strictly speaking - immunotherapy is currently not recommended for mold allergic patients. First, mold allergen extracts remain ill-defined mixtures of allergenic and non-allergenic components. They contain a very complex spectrum of proteins, glycoproteins, carbohydrates, and other components that definitely do not contribute to the allergenicity of the whole extract but might lead to impaired diagnostic tests or to side effects during treatment. Second, the selection of patients for immunotherapy is impaired by the fact that many mould allergic patients suffer from asthma and are sensitized to other common inhalant allergens.54 Although immunotherapy has been shown to be effective for the treatment of allergic asthma, especially this group of patients is prone to develop adverse reactions during treatment. Therefore, clinical manifestations of asthma have been considered rather as a contraindication for immunotherapy.115 Nevertheless, studies with mold extracts, predominately Alternaria and Cladosporium species, have been performed (Table 1). We found 6 studies which evaluated the safety of immunotherapy using mold extracts.116,117,118,119,120,121 In 1982, Kaad and Ostergaard treated 38 children with an Alternaria iridis extract mixed with a Cladosporium herbarum extract over a period of 3.5 years. Nineteen percent of the patients had to stop treatment because of severe side reactions associated with the generation of precipitating serum antibodies.116 Four years later, the same authors reported more severe and even anaphylactic reactions to Alternaria and Cladosporium extracts than to extracts of grass pollen, animal dander, or house dust mite in asthmatic children resistant to pharmacological treatment. Since 50% of patients had to be withdrawn from treatment, the authors concluded that immunotherapy in asthmatics with mold allergy is not recommended.117 These results were further confirmed by a retrospective study with 83 adults and 46 children with rhinitis and/or bronchial asthma. Using a biologically standardized depot extract of Alternaria tenuis, 39.5% of patients experienced adverse reactions which were mainly systemic. Children and patients with asthma were at higher risk of developing side effects.119 Moreno et al.120 investigated the safety of 4 different induction schedules in 108 patients (see Table 1). Only a few adverse reactions to an extract of Alternaria tenuis were reported which were associated with only 1 induction schedule. Finally, Martínez-Canavate et al.121 compared the tolerance of a conventional short regime to a cluster regime in a pediatric population. Whereas more local reactions were observed in the conventional regime, no significant differences between the 2 regimes were found regarding systemic reactions. In total, 10 (10.6%) out of 94 patients had local or systemic reactions and 1 patient from the cluster regime had to be withdrawn from treatment.
Based on the high incidence of adverse reactions, mold extracts were stated as "less tolerated" than other allergen extracts.117,119 Therefore, only a few double-blind, placebo controlled studies have been performed so far that analyze the clinical and immunological efficacy of mold immunotherapy. In 1986, Dreborg et al.122 treated 16 children actively over a period of 10 months with a standardized Cladosporium herbarum extract, while 14 children received a histamine placebo. All the children were polysensitized and suffered from rhinoconjunctivits and/or mold-induced asthma. Symptom scores and total medication score remained unchanged in both groups after 6 months of treatment. Although skin reactivity, conjunctival sensitivity, and peak expiratory flow improved, no significant differences were found between the actively treated and placebo groups. However, a significant reduction in bronchial and conjunctival sensitivity as well as a significant decrease in specific IgE antibodies could be observed in the extract-treated group after 10 months of treatment. A drawback was the high frequency of systemic side effects, which occurred in 81% of patients, especially during the peak of mold season.122,123 In a similar approach with Cladosporium herbarum, the efficacy of immunotherapy was evaluated in 11 adult asthmatics. After 5 to 7 months of treatment, no differences were found between the active and placebo-treated groups when the symptom and medication score were evaluated separately. A combined evaluation revealed that 81% of the C. herbarum treated group and 27% of the placebo control group had unchanged or improved symptoms.124 A significant decrease in bronchial and dermal sensitivity as well as a significant increase in specific IgG1 and IgG4 antibodies was observed in the actively treated group.125,126 Furthermore, a significant decrease in histamine release was reported from basophils isolated from patients of the actively treated group.127 Local side effects were reported in 70% and systemic reactions in 100% of the C. herbarum treated group.124 Immunotherapy with a standardized extract of Alternaria alternata resulted in an improved symptom-medication score, an increased tolerance in nasal provocation tests, and reduced skin reactivity in the actively treated group. Specific IgG antibodies increased significantly after 1 year of treatment, specific IgE antibodies did not change in either group.128 Criado Molina et al.129 evaluated the efficacy and safety of immunotherapy with Alternaria alternata in a sublingual approach. Nineteen out of 38 patients received over a period of 12 months oral immunotherapy, whereas the control group was symptomatically treated. Eight adverse reactions occurred that were described as mild to moderate. Skin and bronchial reactivity were significantly improved in the actively treated group, and an increase in specific IgG4 antibodies was observed. Peak expiratory flow and specific IgE antibody levels remained unchanged in both groups. Another double-blind, placebo-controlled study was carried out in 2008 and included 28 patients. Fourteen patients were treated with a conventional schedule of subcutaneous immunotherapy, the control group received a histamine placebo. The allergen extract applied was a biologically standardized preparation of Alternaria alternata. The highest maintenance dose that induces no systemic side reactions was evaluated in an earlier study before the immunotherapy trial.130 Over a period of 12 months, no local but 2 systemic reactions were reported in asthmatic patients. The major significant improvements for the Alternaria-treated group were an increase in peak expiratory flow and a decrease in severity of asthma.131 No changes in nasal or ocular symptoms as well as in medication scores could be observed. Allergen-specific IgE antibodies decreased, whereas antibody titers of total and specific IgG (IgG1 and IgG4) increased.132 A double-blind, placebo-controlled study included 50 children and adolescents (30 active group, 20 placebo group) with A. alternata-related seasonal allergic rhinoconjunctivitis and/or asthma. Standardized A. alternata extract were injected over 3 years according to the conventional schedule of subcutaneous immunotherapy. A group that received active therapy resulted in improved quality of life regarding asthma and decreased sensitivity upon nasal challenge. The combined symptom medication score decreased in years 2 (38.7%) and 3 (63.5%) of the study. No severe side effects were observed while mild local reactions were reported in 7 patients.133
Besides these double-blind, placebo-controlled studies, a few open studies have been performed. Thirty-nine children with rhinitis and/or Alternaria-induced asthma were enrolled together with 40 age-matched controls in a prospective immunotherapy study. After 3 years of therapy, all patients in the active group reported clinical improvement in a questionnaire, while the symptoms worsened in 87.5% of control patients. The clinical effect seemed to be dose-dependent as patients receiving higher cumulative doses improved considerably.134 Bernardis et al.39 compared the efficacy of subcutaneous immunotherapy (SIT) to sublingual immunotherapy (SLIT) using an extract of Alternaria tenuis. Eleven patients were randomized to the SIT group and 12 to the SLIT group. Although SLIT was very well tolerated, the SIT approach seemed to be more efficient. In both approaches, patients subjectively reported an improvement in symptom medication scores. The threshold to elicit allergic symptoms by nasal provocation test increased in both groups but reached only statistically significance in the SLIT group. However, skin reactivity and IgE levels significantly decreased only in the SIT group, whereas total IgG and specific IgG4 levels increased. Nevertheless, side effects occurred in approximately 36.4% of patients treated with SIT. Recently, Kilic et al.135 evaluated efficacy of SIT in 16 children with bronchial asthma monosensitized to Alternaria in a prospective, open parallel group-controlled study. After 1 year of treatment with Alternatia extracts, a reduction in bronchial responsiveness, a reduction in eosinophil counts in sputum and a decrease in specific IgE levels were found in the actively treated group. In summary, improvements in symptoms have been observed in SIT studies for mold allergy and one can therefore assume that SIT should be effective for mold allergy as it is for many other allergens. However, progress seems to be limited by natural allergen extracts which induce side effects and are of poor quality. It will therefore be necessary to use recombinant mold allergen derivatives with reduced allergenic activity or mold allergen-derived peptides for treatment.
Available data suggests that the insufficient quality of natural mold allergen extracts is the major bottleneck for the development of safe and effective SIT for mold allergy. Especially during the SIT of mold allergy, the occurrence of local and severe systemic side reactions was found to hamper the application of sufficiently high allergen doses needed for efficient therapy (see Table 1). Improvements are therefore needed that allow the safe administration of sufficiently high doses. Furthermore, all disease-relevant allergens must be included in vaccines which often cannot be achieved with allergen extracts that lack certain allergens.
The cDNA sequences of many mold allergens have already been elucidated (see supplementary Table 1), and by using various expression systems recombinant molecules can be produced in large quantities and high purity. Recombinant wilde-type allergens, which resemble immunologically their natural counterpart, represent valuable tools for specific diagnosis, as they can be used in diagnostic tests to determine the disease-causing allergen source. Using component-resolved diagnosis, relevant recombinant allergens can be selected for allergen SIT. The feasibility of this approach has already been shown in case of grass pollen and birch pollen allergens, but so far recombinant allergens have not yet been evaluated for the treatment of mould allergies.
However, when the use of recombinant wild-type allergens is considered for SIT, one has to be aware that the administration of a recombinant allergen mimicking the characteristics of the natural allergen carries the risk of inducing allergic reactions similar as with allergen extracts. Adverse reaction might particularly pose a problem in case of mold allergy, as many patients suffer from severe symptoms. Therefore, the development of genetically modified allergen variants with reduced allergenic activity (hypoallergenic derivatives) represents a desirable approach.136,137
The main strategy behind recombinant hypoallergens is the elimination of IgE reactivity while retaining T-cell reactivity. The disruption of IgE-binding epitopes can be achieved by (1) introduction of point mutations, (2) deletion of IgE reactive parts, (3) fragmentation of allergens, (4) reassembling of allergens in the form of mosaic molecules, (5) oligomerisation of allergens, and (6) chemical denaturation of allergens.138 Hypoallergenic derivatives exhibit a reduced or even abolished IgE reactivity and hence do not induce IgE-mediated side effects when administered to the patient. Immunologically, they may induce T-cell modulatory activities and the production of blocking antibodies due to the presence of T-cell and B-cell epitopes.
In addition, there are currently 2 strategies based on the use of allergen-derived peptides under investigation, which differ fundamentally in their immunological mode of action: a T-cell and a B-cell epitope approach. Regarding the first, T cell epitope-containing peptides (10-12 amino acids) are designed to lack IgE-binding epitopes and are thought to act via the induction of tolerance or anergy. Administration of such peptides in clinical studies proved the lack of IgE-mediated adverse events, but a drawback was the occurrence of late-phase reactions.139,140 For the second approach, peptides (20-40 amino acids) derived from IgE-binding sites on the surface of the allergen are taken and fused with an allergen-unrelated carrier molecule with the aim to induce upon immunization IgG antibodies which are directed toward IgE-binding sites on the allergen and hence block IgE binding to the natural allergen.72,141 They rely on the principle that peptides which are taken from the IgE-binding sites themselves do not react with IgE and therefore are unable to trigger IgE-mediated side effects. The peptides are also selected to contain as little as possible remaining allergen-specific T cell epitopes to also avoid the induction of late-phase reactions due to activation of allergen-specific T cells upon administration to the patient.142,143 To induce allergen-specific IgG, the allergen-derived peptides are bound to carrier molecules that provide T-cell help. In the beginning peptides were coupled chemically to KLH (keyhole limpet hemocyanin), a protein from the mollusc Megathura crenulata but the vaccines which are currently in clinical trial are based on recombinant fusion proteins consisting of a viral carrier proteins which are fused to the allergen peptides to facilitate reproducible large-scale production under good manufacturing practice (GMP).141,144 The latter strategy holds promise that vaccines can be developed which can be given as few high dose injections (3-4 per year) to patients without inducing severe systemic side effects and therefore may be particularly suitable for the treatment of mold-sensitized patients, who often suffer from severe symptoms (e.g., asthma) and are at high risk of developing adverse reactions.
Although fungi have been underestimated allergen sources, the sequences of an increasing number of mold allergens are becoming available and in particular the major allergens from some of the most important allergenic molds have been characterized.5,6 Also our knowledge of IgE and T-cell epitopes of mold allergens increases. By using short synthetic peptides, IgE-binding epitopes of Alt a 1,145,146 Asp f 2, Cla h 6, Pen ch 13,147 and Pen n 18148 have been determined. Based on this information, hypoallergens or hypoallergenic derivatives can be developed. For example, deletion of amino acids from the N- and C-terminus of Asp f 2 abrogated IgE binding in sera from Aspergillus-sensitized patients.149 A lower IgE reactivity was also obtained by site-directed mutagenesis of the Malassezia sympodialis allergen Mala s 11150 and the Penicillium crysogenum allergen Pen ch 18.151 The insertion of 2 mutations in an IgE-reactive fragment of Alt a 13 resulted in a mutant with reduced IgE binding and reduced allergenic activity. Stimulation of PBMCs from Alternaria-sensitized patients with this mutant revealed a retained T-cell reactivity with reduced production of IL-4.152 We recently identified Alt a 1-derived peptides without allergenic activity, but with ability to induce Alt a 1 specific IgGs that could inhibit allergic patients' IgE binding to Alt a 1.72 Based on these results it should be possible to construct non-allergenic fusion proteins consisting of the peptides and a suitable carrier protein for the development of a safe candidate vaccine for SIT of Alternaria allergy which then can be evaluated in clinical studies. In order to advance progress in the field of mold allergy to other allergenic molds, it will be also important to identify the most relevant mold genera and their clinically relevant allergens in population studies so that the prerequisites for vaccine development are available.
Figures and Tables
Fig. 1
Seasonal variations influence the number of spores in the atmosphere. The climatic conditions in summer and autumn favour the growth of certain fungal species and the dispersal of spores. Exceptional high concentrations of spores from the species Alternaria and Cladosporium as well as from species of the phylum Basidiomycota can be found from June to October (modified after Lacey J, 1996).

Fig. 2
Geographical distribution of fungal allergy. Fungal allergy represents a worldwide health problem. The world map highlights in yellow all countries in which sensitization to fungi has been described.
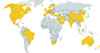
Fig. 3
Fungal species as a source of type I allergy. The taxonomical tree includes all allergen producing fungal species that have been approved by the I.U.I.S. Allergen Nomenclature Sub-committee (http://www.allergen.org).
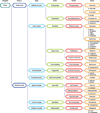
Fig. 4
Fungal spore seasons overlap with grass and weed pollen seasons. Diagnosis of fungal allergy is complicated by the fact that mould allergic patients are often polysensitized. Exacerbated allergic symptoms in summer and autumn might be due to fungal allergy, but can also arise from allergy to grasses and weeds like mugwort (Artemisia), ragweed (Ambrosia) or Parietaria. The intensity of allergen exposure to grasses, weeds and moulds is displayed in red for high exposure, orange for intermediate exposure and yellow for low exposure.

Fig. 5
Influence of strain variabilities and growth conditions on the presence of IgE binding proteins in extracts of Alternaria alternata. Four Alternaria strains, achieved by the Centraalbureau voor Schimmelcultures (CBS 103.33, CBS 795.72) and the American Type Culture Collection (ATCC 46582 and ATCC 96154) were grown on 3 different media (C, Czapek Dox agar; ME, Malt Extract agar; S, Sabauroud agar) for 2 or 4 weeks respectively. Protein extracts of the fungi were subjected to SDS-PAGE, blotted onto nitrocellulose membranes and incubated with the serum of an Alternaria-sensitized patient. Bound IgE antibodies were detected with 125I-labelled anti-human IgE antibodies and visualized by autoradiography
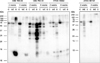
Table 1A
Immunotherapy studies with fungal extracts

| Design of study | Mould species | Schedule | No. of patients | Side-effects | Withdrawal due to side-effects | Ref. | |
|---|---|---|---|---|---|---|---|
| Local | Systemic | ||||||
| Retrospective | Alternaria alternata | SIT, cluster | 52 | 1 | 3 | 1 | [121] |
| SIT, conventional short | 42 | 4 | 2 | ||||
| Retrospective | Alternaria tenuis | SIT, cluster | 108 | 0 | [120] | ||
| 4 visits/10 injections | 60 | 1 | 2 | ||||
| 4 visits/8 injections | 9 | 0 | 0 | ||||
| 3 visits/9 injections | 6 | 0 | 0 | ||||
| 3 visits/6 injections | 33 | 0 | 0 | ||||
| Retrospective | Alternaria tenuis | SIT, conventional | 129 | 3 | 48 | n.k. | [119] |
| Prospective | Alternaria iridis/alternata | SIT, conventional | 13 | n.k. | n.k. | 4 | [117] |
| Cladosporium herbarum | 13 | n.k. | n.k. | 9 | |||
| Prospective | Alternaria iridis mixed with Cladosporium herbarum | SIT, conventional | 38 | n.k. | n.k. | 7 | [116] |
Table 1B
Immunotherapy studies with fungal extracts

| Design of study | Mould species | Schedule | No. of patients active/placebo | Clinical effect | Immunological effects | Side-effects | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Local | Systemic | |||||||
| DB, PC | C. herbarum | SIT, cluster | 16/14 | medication score↓ | IgE↓, IgG↑ | 4* | 13 | [122,123] |
| bronchial sensitivity↓ | ||||||||
| DB, PC | C. herbarum | SIT, cluster | 11/11 | symptom medication score↓ | IgE↓ | 8 | 11 | [124,125,126,127] |
| PEF↑ | IgG1 and IgG4↑ | |||||||
| skin reactivity↓ | ||||||||
| DB, PC | Alternaria sp. | SIT, rush | 13/11 | global symptom medication score↓ | IgG↑ | 0 | 2 | [128] |
| skin reactivity↓ | ||||||||
| nasal tolerance↑ | ||||||||
| DB, PC | A. alternata | SLIT | 19/20 | symptom medication score↓ | IgG4↑ | 8 | [129] | |
| skin reactivity↓ | ||||||||
| respiratory tolerance↑ (challenge) | ||||||||
| DB, PC | A. alternata | SIT, conventional | 14/14 | conjunctival sensitivity↓ | IgE↓ | 0 | 2 | [131,132] |
| PEF↑ | IgG, IgG1 and IgG4↑ | |||||||
| DB, PC | A. alternata | SIT, conventional | 30/20 | symptom medication score↓ | IgE↓ | 7 | 0 | [133] |
| nasal tolerance↑ | ||||||||
| Case control | A. tenuis | SIT, conventional | 39/40 | symptom score↓ | n.k. | + | 0 | [134] |
| medication score↓ | ||||||||
| Open control | A. tenuis | SIT, conventional | 11 | skin reactivity↓ | IgE↓ | 2 | 2 | [39] |
| symtom score↓ | IgG and IgG4↑ | |||||||
| medication score↓ | ||||||||
| SLIT | 12 | symtom score↓ | 0 | 0 | [39] | |||
| medication score↓ | ||||||||
| nasal tolerance↑ | ||||||||
| Open control | A. alternata | SIT, conventional | 9/7 | bronchial sensitivity↓ | IgE↓ | [135] | ||
*number of reactions.
n.k., not known; DB, PC, double blind placebo-controlled; C. herbarum, Cladosporium herbarum; Alternaria sp., Alternaria species; A. alternata, Alternaria alternata; A. tenuis, Alternaria tenuis; SIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; PEF, peak expiratory flow.
ACKNOWLEDGMENTS
This work was supported by a research grant from Biomay AG, Vienna, Austria and by a grant F4605 of the Austrian Science Fund (FWF).
References
1. von Pirquet C. Allergie. Münch Med Wochenschr. 1906; 53:1457–1458.
2. Floyer J. Violent asthma after visiting a wine cellar. London: Innys and Parker;1745.
3. Blackley C. Experimental research on the cause and nature of Catarrhus Aestivus (hay fever or hay asthma). London: Bailliere, Tindall and Cox;1873.
4. Newson R, Strachan D, Corden J, Millington W. Fungal and other spore counts as predictors of admissions for asthma in the Trent region. Occup Environ Med. 2000; 57:786–792.
5. Crameri R, Garbani M, Rhyner C, Huitema C. Fungi: the neglected allergenic sources. Allergy. 2014; 69:176–185.
6. Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008; 145:58–86.
7. Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F. European Community Respiratory Health Survey. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. Bmj. 2002; 325:411–414.
8. Jo EJ, Kim MY, Lee SE, Lee SY, Kim MH, Song WJ, et al. Eosinophilic airway inflammation and airway hyperresponsiveness according to aeroallergen sensitization pattern in patients with lower airway symptoms. Allergy Asthma Immunol Res. 2014; 6:39–46.
9. Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, et al. Mold exposure during infancy as a predictor of potential asthma development. Ann Allergy Asthma Immunol. 2009; 102:131–137.
10. Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin Exp Allergy. 1998; 28:459–467.
11. Nolles G, Hoekstra MO, Schouten JP, Gerritsen J, Kauffman HF. Prevalence of immunoglobulin E for fungi in atopic children. Clin Exp Allergy. 2001; 31:1564–1570.
12. Andriessen JW, Brunekreef B, Roemer W. Home dampness and respiratory health status in european children. Clin Exp Allergy. 1998; 28:1191–1200.
13. Hagmolen of Ten Have W, van den Berg NJ, van der Palen J, van Aalderen WM, Bindels PJ. Residential exposure to mould and dampness is associated with adverse respiratory health. Clin Exp Allergy. 2007; 37:1827–1832.
14. Meng J, Barnes CS, Rosenwasser LJ. Children's Mercy Center for Environmental Health. Identity of the fungal species present in the homes of asthmatic children. Clin Exp Allergy. 2012; 42:1448–1458.
15. Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J Allergy Clin Immunol. 1995; 95:955–961.
16. Rodriguez-Rajo FJ, Iglesias I, Jato V. Variation assessment of airborne Alternaria and Cladosporium spores at different bioclimatical conditions. Mycol Res. 2005; 109:497–507.
17. Hamilton ED. Pollen and fungus spore counts. Proc R Soc Med. 1963; 56:220–221.
18. Lacey J. Spore dispersal - its role in ecology and disease: the British contribution to fungal aerobiology. Mycol Res. 1996; 100:641–660.
19. Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007; 120:610–617.
20. Hoffman DR, Kozak PP Jr, Gillman SA, Cummins LH, Gallup J. Isolation of spore specific allergens from Alternaria. Ann Allergy. 1981; 46:310–316.
21. Wahl R, Oliver JD, Hauck PR, Winter HG, Maasch HJ. Comparison of Alternaria tenuis extracts prepared from different raw materials. Ann Allergy. 1989; 63:527–531.
22. Paris S, Fitting C, Latgé JP, Herman D, Guinnepain MT, David B. Comparison of conidial and mycelial allergens of Alternaria alternata. Int Arch Allergy Appl Immunol. 1990; 92:1–8.
23. Fadel R, David B, Paris S, Guesdon JL. Alternaria spore and mycelium sensitivity in allergic patients: in vivo and in vitro studies. Ann Allergy. 1992; 69:329–335.
24. Licorish K, Novey HS, Kozak P, Fairshter RD, Wilson AF. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J Allergy Clin Immunol. 1985; 76:819–825.
25. Matsuwaki Y, Uno K, Okushi T, Otori N, Moriyama H. Total and antigen- (fungi, mites and staphylococcal enterotoxins) specific IgEs in nasal polyps is related to local eosinophilic inflammation. Int Arch Allergy Immunol. 2013; 161:Suppl 2. 147–153.
26. Niedoszytko M, Chełmińska M, Chełmiński K, Knopińska-Posłuszny W, Gruchała-Niedoszytko M, Jassem E. Late-phase allergic reaction in nasal provocation with fungal allergens. Allergy Asthma Proc. 2008; 29:35–39.
27. Paris S, Debeaupuis JP, Prévost MC, Casotto M, Latgé JP. The 31 kd major allergen, Alt a I1563, of Alternaria alternata. J Allergy Clin Immunol. 1991; 88:902–908.
28. Ibarrola I, Suárez-Cervera M, Arilla MC, Martínez A, Monteseirín J, Conde J, et al. Production profile of the major allergen Alt a 1 in Alternaria alternata cultures. Ann Allergy Asthma Immunol. 2004; 93:589–593.
29. Twaroch TE, Arcalís E, Sterflinger K, Stöger E, Swoboda I, Valenta R. Predominant localization of the major Alternaria allergen Alt a 1 in the cell wall of airborne spores. J Allergy Clin Immunol. 2012; 129:1148–1149.
30. Rivera-Mariani FE, Vysyaraju K, Negherbon J, Levetin E, Horner WE, Hartung T, et al. Comparison of the interleukin-1β-inducing potency of allergenic spores from higher fungi (basidiomycetes) in a cryopreserved human whole blood system. Int Arch Allergy Immunol. 2014; 163:154–162.
31. Wada K, Kobayashi T, Matsuwaki Y, Moriyama H, Kita H. Alternaria inhibits double-stranded RNA-induced cytokine production through Toll-like receptor 3. Int Arch Allergy Immunol. 2013; 161:Suppl 2. 75–83.
32. Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014; 163:92–105.
33. D'Amato G, Spieksma FT. Aerobiologic and clinical aspects of mould allergy in Europe. Allergy. 1995; 50:870–877.
34. Oh SY, Fong JJ, Park MS, Chang L, Lim YW. Identifying airborne fungi in Seoul, Korea using metagenomics. J Microbiol. 2014; 52:465–472.
35. Fröhlich-Nowoisky J, Pickersgill DA, Després VR, Pöschl U. High diversity of fungi in air particulate matter. Proc Natl Acad Sci U S A. 2009; 106:12814–12819.
36. Kilic M, Ufuk Altintas D, Yilmaz M, Güneşer Kendirli S, Bingöl Karakoc G, Taskin E, et al. The effects of meteorological factors and Alternaria spore concentrations on children sensitised to Alternaria. Allergol Immunopathol (Madr). 2010; 38:122–128.
37. Şakiyan N, Inceoglu Ö. Atmospheric concentrations of Cladosporium Link and Alternaria Nées spores in Ankara and the effects of meteorological factors. Turk J Bot. 2003; 27:77–81.
38. Bowyer P, Fraczek M, Denning DW. Comparative genomics of fungal allergens and epitopes shows widespread distribution of closely related allergen and epitope orthologues. BMC Genomics. 2006; 7:251.
39. Bernardis P, Agnoletto M, Puccinelli P, Parmiani S, Pozzan M. Injective versus sublingual immunotherapy in Alternaria tenuis allergic patients. J Investig Allergol Clin Immunol. 1996; 6:55–62.
40. Green BJ, Yli-Panula E, Tovey ER. Halogen immunoassay, a new method for the detection of sensitization to fungal allergens; comparisons with conventional techniques. Allergol Int. 2006; 55:131–139.
41. Cho SH, Seo SC, Schmechel D, Grinshpun SA, Reponen T. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ. 2005; 39:5454–5465.
42. Barnes C, Schreiber K, Pacheco F, Landuyt J, Hu F, Portnoy J. Comparison of outdoor allergenic particles and allergen levels. Ann Allergy Asthma Immunol. 2000; 84:47–54.
43. Feo Brito F, Alonso AM, Carnés J, Martín-Martín R, Fernández-Caldas E, Galindo PA, et al. Correlation between Alt a 1 levels and clinical symptoms in Alternaria alternata-monosensitized patients. J Investig Allergol Clin Immunol. 2012; 22:154–159.
44. Mitakakis TZ, Barnes C, Tovey ER. Spore germination increases allergen release from Alternaria. J Allergy Clin Immunol. 2001; 107:388–390.
45. Heinzerling L, Frew AJ, Bindslev-Jensen C, Bonini S, Bousquet J, Bresciani M, et al. Standard skin prick testing and sensitization to inhalant allergens across Europe--a survey from the GALEN network. Allergy. 2005; 60:1287–1300.
46. Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014; 6:222–227.
47. Lyons TW, Wakefield DB, Cloutier MM. Mold and Alternaria skin test reactivity and asthma in children in Connecticut. Ann Allergy Asthma Immunol. 2011; 106:301–307.
48. Smit LA, Bouzigon E, Bousquet J, Le Moual N, Nadif R, Pin I, et al. Epidemiological Study on the Genetics and Environment of Asthma. Mold allergen sensitization in adult asthma according to integrin β3 polymorphisms and Toll-like receptor 2/+596 genotype. J Allergy Clin Immunol. 2011; 128:185–191.
49. Wiszniewska M, Tymoszuk D, Nowakowska-Świrta E, Pałczyński C, Walusiak-Skorupa J. Mould sensitisation among bakers and farmers with work-related respiratory symptoms. Ind Health. 2013; 51:275–284.
50. D'Amato G, Chatzigeorgiou G, Corsico R, Gioulekas D, Jäger L, Jäger S, et al. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. A European multicenter study promoted by the Subcommittee on Aerobiology and Environmental Aspects of Inhalant Allergens of the European Academy of Allergology and Clinical Immunology. Allergy. 1997; 52:711–716.
51. de Benedictis FM, Franceschini F, Hill D, Naspitz C, Simons FE, Wahn U, et al. EPAAC Study Group. The allergic sensitization in infants with atopic eczema from different countries. Allergy. 2009; 64:295–303.
52. Arbes SJ Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005; 116:377–383.
53. Rivera-Mariani FE, Nazario-Jiménez S, López-Malpica F, Bolaños-Rosero B. Skin test reactivity of allergic subjects to basidiomycetes' crude extracts in a tropical environment. Med Mycol. 2011; 49:887–891.
54. Mari A, Schneider P, Wally V, Breitenbach M, Simon-Nobbe B. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin Exp Allergy. 2003; 33:1429–1438.
55. Niemeijer NR, de Monchy JG. Age-dependency of sensitization to aero-allergens in asthmatics. Allergy. 1992; 47:431–435.
56. Ezeamuzie CI, Al-Ali S, Khan M, Hijazi Z, Dowaisan A, Thomson MS, et al. IgE-mediated sensitization to mould allergens among patients with allergic respiratory diseases in a desert environment. Int Arch Allergy Immunol. 2000; 121:300–307.
57. Bartra J, Belmonte J, Torres-Rodriguez JM, Cistero-Bahima A. Sensitization to Alternaria in patients with respiratory allergy. Front Biosci. 2009; 14:3372–3379.
58. Moral L, Roig M, Garde J, Alós A, Toral T, Fuentes MJ. Allergen sensitization in children with asthma and rhinitis: marked variations related to age and microgeographical factors. Allergol Immunopathol (Madr). 2008; 36:128–133.
59. Cantani A, Ciaschi V. Epidemiology of alternaria alternata allergy: a prospective study in 6840 Italian asthmatic children. Eur Rev Med Pharmacol Sci. 2004; 8:289–294.
60. Niederberger V, Stübner P, Spitzauer S, Kraft D, Valenta R, Ehrenberger K, et al. Skin test results but not serology reflect immediate type respiratory sensitivity: a study performed with recombinant allergen molecules. J Invest Dermatol. 2001; 117:848–851.
61. Liang KL, Su MC, Jiang RS. Comparison of the skin test and ImmunoCAP system in the evaluation of mold allergy. J Chin Med Assoc. 2006; 69:3–6.
62. Kespohl S, Maryska S, Zahradnik E, Sander I, Brüning T, Raulf-Heimsoth M. Biochemical and immunological analysis of mould skin prick test solution: current status of standardization. Clin Exp Allergy. 2013; 43:1286–1296.
63. Soeria-Atmadja D, Onell A, Borgå A. IgE sensitization to fungi mirrors fungal phylogenetic systematics. J Allergy Clin Immunol. 2010; 125:1379–1386.
64. Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010; 28:211–241.
65. Limacher A, Kloer DP, Flückiger S, Folkers G, Crameri R, Scapozza L. The crystal structure of Aspergillus fumigatus cyclophilin reveals 3D domain swapping of a central element. Structure. 2006; 14:185–195.
66. Glaser AG, Limacher A, Flückiger S, Scheynius A, Scapozza L, Crameri R. Analysis of the cross-reactivity and of the 1.5 A crystal structure of the Malassezia sympodialis Mala s 6 allergen, a member of the cyclophilin pan-allergen family. Biochem J. 2006; 396:41–49.
67. Limacher A, Glaser AG, Meier C, Schmid-Grendelmeier P, Zeller S, Scapozza L, et al. Cross-reactivity and 1.4-A crystal structure of Malassezia sympodialis thioredoxin (Mala s 13), a member of a new pan-allergen family. J Immunol. 2007; 178:389–396.
68. Nüss D, Goettig P, Magler I, Denk U, Breitenbach M, Schneider PB, et al. Crystal structure of the NADP-dependent mannitol dehydrogenase from Cladosporium herbarum: Implications for oligomerisation and catalysis. Biochimie. 2010; 92:985–993.
69. Chruszcz M, Chapman MD, Osinski T, Solberg R, Demas M, Porebski PJ, et al. Alternaria alternata allergen Alt a 1: a unique β-barrel protein dimer found exclusively in fungi. J Allergy Clin Immunol. 2012; 130:241–247.
70. Wagner GE, Gutfreund S, Fauland K, Keller W, Valenta R, Zangger K. Backbone resonance assignment of Alt a 1, a unique β-barrel protein and the major allergen of Alternaria alternata. Biomol NMR Assign. 2014; 8:229–231.
71. Chen JC, Chiu LL, Lee KL, Huang WN, Chuang JG, Liao HK, et al. Identification of critical amino acids in an immunodominant IgE epitope of Pen c 13, a major allergen from Penicillium citrinum. PLoS One. 2012; 7:e34627.
72. Twaroch TE, Focke M, Fleischmann K, Balic N, Lupinek C, Blatt K, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin Exp Allergy. 2012; 42:966–975.
73. Nair S, Kukreja N, Singh BP, Arora N. Identification of B cell epitopes of alcohol dehydrogenase allergen of Curvularia lunata. PLoS One. 2011; 6:e20020.
74. Herrera-Mozo I, Ferrer B, Luís Rodriguez-Sanchez J, Juarez C. Description of a novel panallergen of cross-reactivity between moulds and foods. Immunol Invest. 2006; 35:181–197.
75. Simon-Nobbe B, Probst G, Kajava AV, Oberkofler H, Susani M, Crameri R, et al. IgE-binding epitopes of enolases, a class of highly conserved fungal allergens. J Allergy Clin Immunol. 2000; 106:887–895.
76. Chang CY, Chou H, Tam M, Tang RB, Lai HY, Shen HD. Characterization of enolase allergen from Rhodotorula mucilaginosa. J Biomed Sci. 2002; 9:645–655.
77. Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Detection of IgE antibody against Candida albicans enolase and its crossreactivity to Saccharomyces cerevisiae enolase. Clin Exp Allergy. 1995; 25:522–528.
78. Flückiger S, Scapozza L, Mayer C, Blaser K, Folkers G, Crameri R. Immunological and structural analysis of IgE-mediated cross-reactivity between manganese superoxide dismutases. Int Arch Allergy Immunol. 2002; 128:292–303.
79. Horner WE, Reese G, Lehrer SB. Identification of the allergen Psi c 2 from the basidiomycete Psilocybe cubensis as a fungal cyclophilin. Int Arch Allergy Immunol. 1995; 107:298–300.
80. Flückiger S, Fijten H, Whitley P, Blaser K, Crameri R. Cyclophilins, a new family of cross-reactive allergens. Eur J Immunol. 2002; 32:10–17.
81. Shankar J, Gupta PD, Sridhara S, Singh BP, Gaur SN, Arora N. Immunobiochemical analysis of cross-reactive glutathione-S-transferase allergen from different fungal sources. Immunol Invest. 2005; 34:37–51.
82. Glaser AG, Menz G, Kirsch AI, Zeller S, Crameri R, Rhyner C. Auto- and cross-reactivity to thioredoxin allergens in allergic bronchopulmonary aspergillosis. Allergy. 2008; 63:1617–1623.
83. Chou H, Wu KG, Yeh CC, Tai HY, Tam MF, Chen YS, et al. The Transaldolase, a Novel Allergen of Fusarium proliferatum, Demonstrates IgE Cross-Reactivity with Its Human Analogue. PLoS One. 2014; 9(7):e103488.
84. van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int Arch Allergy Immunol. 2002; 129:189–197.
85. Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007; 142:99–115.
86. Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R, Lawrence CB, et al. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol. 2009; 182:2502–2510.
87. Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, et al. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006; 61:382–388.
88. Borger P, Koëter GH, Timmerman JA, Vellenga E, Tomee JF, Kauffman HF. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 production in airway epithelial cell lines by transcriptional mechanisms. J Infect Dis. 1999; 180:1267–1274.
89. Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000; 105:1185–1193.
90. Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008; 180:6000–6009.
91. Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, et al. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011; 300:L605–L614.
92. Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33 mediated asthma exacerbations. J Allergy Clin Immunol. 2014; 134:583–592.
93. Chen JC, Chuang JG, Su YY, Chiang BL, Lin YS, Chow LP. The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model. J Biol Chem. 2011; 286:26667–26679.
94. Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy. III. Diagnosis of Cladosporium allergy by means of symptom score, bronchial provocation test, skin prick test, RAST, CRIE and histamine release. Allergy. 1986; 41:57–67.
95. Malling HJ, Agrell B, Croner S, Dreborg S, Foucard T, Kjellman M, et al. Diagnosis and immunotherapy of mould allergy. I. Screening for mould allergy. Allergy. 1985; 40:108–114.
96. Palma-Carlos ML, Palma-Carlos AG. "In vivo" and "in vitro": tests in the diagnosis of trichophyton allergy. Eur Ann Allergy Clin Immunol. 2007; 39:328–332.
97. Schumacher MJ, Jeffrey SE. Variability of Alternaria alternata: biochemical and immunological characteristics of culture filtrates from seven isolates. J Allergy Clin Immunol. 1976; 58:263–277.
98. Steringer I, Aukrust L, Einarsson R. Variability of antigenicity/allergenicity in different strains of Alternaria alternata. Int Arch Allergy Appl Immunol. 1987; 84:190–197.
99. Vijay HM, Young NM, Jackson GE, White GP, Bernstein IL. Studies on Alternaria allergens. V. Comparative biochemical and immunological studies of three isolates of Alternaria tenuis cultured on synthetic media. Int Arch Allergy Appl Immunol. 1985; 78:37–42.
100. Portnoy J, Pacheco F, Barnes C, Upadrashta B, Crenshaw R, Esch R. Selection of representative Alternaria strain groups on the basis of morphology, enzyme profile, and allergen content. J Allergy Clin Immunol. 1993; 91:773–782.
101. Martínez J, Gutiérrez A, Postigo I, Cardona G, Guisantes J. Variability of Alt a 1 expression by different strains of Alternaria alternata. J Investig Allergol Clin Immunol. 2006; 16:279–282.
102. Sáenz-de-Santamaría M, Guisantes JA, Martínez J. Enzymatic activities of Alternaria alternata allergenic extracts and its major allergen (Alt a 1). Mycoses. 2006; 49:288–292.
103. Marquardt H. Spontaneous mutations in fungi. Humangenetik. 1972; 16:7–18.
104. Portnoy J, Pacheco F, Ballam Y, Barnes C. The effect of time and extraction buffers on residual protein and allergen content of extracts derived from four strains of Alternaria. J Allergy Clin Immunol. 1993; 91:930–938.
105. Bisht V, Singh BP, Arora N, Sridhara S, Gaur SN. Allergens of Epicoccum nigrum grown in different media for quality source material. Allergy. 2000; 55:274–280.
106. Vailes L, Sridhara S, Cromwell O, Weber B, Breitenbach M, Chapman M. Quantitation of the major fungal allergens, Alt a 1 and Asp f 1, in commercial allergenic products. J Allergy Clin Immunol. 2001; 107:641–646.
107. Brander KA, Borbély P, Crameri R, Pichler WJ, Helbling A. IgE-binding proliferative responses and skin test reactivity to Cop c 1, the first recombinant allergen from the basidiomycete Coprinus comatus. J Allergy Clin Immunol. 1999; 104:630–636.
108. Hemmann S, Menz G, Ismail C, Blaser K, Crameri R. Skin test reactivity to 2 recombinant Aspergillus fumigatus allergens in A fumigatus-sensitized asthmatic subjects allows diagnostic separation of allergic bronchopulmonary aspergillosis from fungal sensitization. J Allergy Clin Immunol. 1999; 104:601–607.
109. Unger A, Stöger P, Simon-Nobbe B, Susani M, Crameri R, Ebner C, Hintner H, Breitenbach M. Clinical testing of recombinant allergens of the mold Alternaria alternata. Int Arch Allergy Immunol. 1999; 118:220–221.
110. Postigo I, Gutiérrez-Rodríguez A, Fernández J, Guisantes JA, Suñén E, Martínez J. Diagnostic value of Alt a 1, fungal enolase and manganese-dependent superoxide dismutase in the component-resolved diagnosis of allergy to Pleosporaceae. Clin Exp Allergy. 2011; 41:443–451.
111. Katotomichelakis M, Anastassakis K, Gouveris H, Tripsianis G, Paraskakis E, Maroudias N, et al. Clinical significance of Alternaria alternata sensitization in patients with allergic rhinitis. Am J Otolaryngol. 2012; 33:232–238.
112. Bousquet J, Lockey R, Malling HJ, Alvarez-Cuesta E, Canonica GW, Chapman MD, et al. World Health Organization. American academy of Allergy, Asthma and Immunology. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Ann Allergy Asthma Immunol. 1998; 81:401–405.
113. Vermani M, Vijayan VK, Kausar MA, Agarwal MK. Quantification of airborne Aspergillus allergens: redefining the approach. J Asthma. 2010; 47:754–761.
114. Barnes C, Pacheco F, Dhar M, Portnoy J. Alternaria and Cladosporium Fungal Allergen Epitopes are Denatured by Sodium Hypochlorite. World Allergy Organ J. 2009; 2:296–302.
115. Frew AJ. Injection immunotherapy. British Society for Allergy and Clinical Immunology Working Party. BMJ. 1993; 307:919–923.
116. Kaad PH, Ostergaard PA. The hazard of mould hyposensitization in children with asthma. Clin Allergy. 1982; 12:317–320.
117. Ostergaard PA, Kaad PH, Kristensen T. A prospective study on the safety of immunotherapy in children with severe asthma. Allergy. 1986; 41:588–593.
118. Martorell A, Sole A, Diez LV, Belenguer J, Sanz J, Torro MI, Cerda JC, Álvarez V. Inmunoterapia en alergia a hongos. Rev Esp Alergol Inmunol Clin. 1986; 1:188–192.
119. Tabar AI, Lizaso MT, García BE, Echechipía S, Olaguibel JM, Rodríguez A. Tolerance of immunotherapy with a standardized extract of Alternaria tenuis in patients with rhinitis and bronchial asthma. J Investig Allergol Clin Immunol. 2000; 10:327–333.
120. Moreno C, Fernández Távora L, Justicia JL, Cabeza N, Vidal C. Investigation of an adequate immunotherapy induction schedule with an Alternaria tenuis extract. Alergol Inmunol Clin. 2001; 16:133–137.
121. Martínez-Cañavate A, Eseverri JL, Ródenas R, Tabar AI, Gardee J, Torres J, et al. Evaluation of paediatric tolerance to an extract of Alternaria alternata under two treatment regimes. A multicentre study. Allergol Immunopathol (Madr). 2005; 33:138–141.
122. Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double-blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation. I. Clinical results. Allergy. 1986; 41:131–140.
123. Karlsson R, Agrell B, Dreborg S, Foucard T, Kjellman NI, Koivikko A, et al. A double-blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation. II. In vitro results. Allergy. 1986; 41:141–150.
124. Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy. V. Clinical efficacy and side effects of immunotherapy with Cladosporium herbarum. Allergy. 1986; 41:507–519.
125. Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy. VI. IgE-mediated parameters during a one-year placebo-controlled study of immunotherapy with Cladosporium. Allergy. 1987; 42:305–314.
126. Malling HJ, Djurup R. Diagnosis and immunotherapy of mould allergy. VII. IgG subclass response and relation to the clinical efficacy of immunotherapy with Cladosporium. Allergy. 1988; 43:60–70.
127. Malling HJ, Stahl Skov P. Diagnosis and immunotherapy of mould allergy. VIII. Qualitative and quantitative estimation of IgE in Cladosporium immunotherapy. Allergy. 1988; 43:228–238.
128. Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990; 85:460–472.
129. Criado Molina A, Guerra Pasadas F, Daza Muñoz JC, Moreno Aguilar C, Almeda Llamas E, Muñoz Gomariz E, et al. Immunotherapy with an oral Alternaria extract in childhood asthma. Clinical safety and efficacy and effects on in vivo and in vitro parameters. Allergol Immunopathol (Madr). 2002; 30:319–330.
130. Lizaso MT, Martínez A, Asturias JA, Algorta J, Madariaga B, Labarta N, et al. Biological standardization and maximum tolerated dose estimation of an Alternaria alternata allergenic extract. J Investig Allergol Clin Immunol. 2006; 16:94–103.
131. Tabar AI, Lizaso MT, García BE, Gómez B, Echechipía S, Aldunate MT, et al. Double-blind, placebo-controlled study of Alternaria alternata immunotherapy: clinical efficacy and safety. Pediatr Allergy Immunol. 2008; 19:67–75.
132. Lizaso MT, Tabar AI, García BE, Gómez B, Algorta J, Asturias JA, et al. Double-blind, placebo-controlled Alternaria alternata immunotherapy: in vivo and in vitro parameters. Pediatr Allergy Immunol. 2008; 19:76–81.
133. Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. 2011; 127:502–508.
134. Cantani A, Businco E, Maglio A. Alternaria allergy: a three-year controlled study in children treated with immunotherapy. Allergol Immunopathol (Madr). 1988; 16:1–4.
135. Kiliç M, Altintaş DU, Yilmaz M, Bingöl-Karakoç G, Burgut R, Güneşer-Kendirli S. Evaluation of efficacy of immunotherapy in children with asthma monosensitized to Alternaria. Turk J Pediatr. 2011; 53:285–294.
136. Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002; 2:446–453.
137. Linhart B, Valenta R. Vaccines for allergy. Curr Opin Immunol. 2012; 24:354–360.
138. Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012; 30:4328–4335.
139. Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999; 189:1885–1894.
140. Oldfield WL, Kay AB, Larché M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol. 2001; 167:1734–1739.
141. Focke-Tejkl M, Valenta R. Safety of engineered allergen-specific immunotherapy vaccines. Curr Opin Allergy Clin Immunol. 2012; 12:555–563.
142. Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. Faseb J. 2001; 15:2042–2044.
143. Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, et al. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004; 34:1525–1533.
144. Edlmayr J, Niespodziana K, Linhart B, Focke-Tejkl M, Westritschnig K, Scheiblhofer S, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009; 182:6298–6306.
145. Zhang L, Curran IH, Muradia G, De Vouge MW, Rode H, Vijay HM. N-terminus of a major allergen, Alt a I, of Alternaria alternata defined to be an epitope. Int Arch Allergy Immunol. 1995; 108:254–259.
146. Kurup VP, Vijay HM, Kumar V, Castillo L, Elms N. IgE binding synthetic peptides of Alt a 1, a major allergen of Alternaria alternata. Peptides. 2003; 24:179–185.
147. Lai HY, Tam MF, Chou H, Lee SS, Tai HY, Shen HD. Molecular and structural analysis of immunoglobulin E-binding epitopes of Pen ch 13, an alkaline serine protease major allergen from Penicillium chrysogenum. Clin Exp Allergy. 2004; 34:1926–1933.
148. Yu CJ, Chen YM, Su SN, Forouhar F, Lee SH, Chow LP. Molecular and immunological characterization and IgE epitope mapping of Pen n 18, a major allergen of Penicillium notatum. Biochem J. 2002; 363:707–715.
149. Banerjee B, Greenberger PA, Fink JN, Kurup VP. Conformational and linear B-cell epitopes of Asp f 2, a major allergen of Aspergillus fumigatus, bind differently to immunoglobulin E antibody in the sera of allergic bronchopulmonary aspergillosis patients. Infect Immun. 1999; 67:2284–2291.
150. Vilhelmsson M, Glaser AG, Martinez DB, Schmidt M, Johansson C, Rhyner C, et al. Mutational analysis of amino acid residues involved in IgE-binding to the Malassezia sympodialis allergen Mala s 11. Mol Immunol. 2008; 46:294–303.
151. Cheng TT, Tam MF, Chou H, Tai HY, Shen HD. Lys89, Lys90, and Phe91 are critical core amino acid residues of the Pen ch 18 major fungal allergen recognized by human IgE antibodies. Biochem Biophys Res Commun. 2008; 375:671–674.
152. Shankar J, Singh BP, Gaur SN, Arora N. Engineered Alt a 13 fragment of Alternaria alternata abrogated IgE binding without affecting T-cell stimulation. J Clin Immunol. 2009; 29:63–70.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download