Abstract
Temozolomide is an oral alkylating agent indicated for the treatment of patients with glioblastoma multiforme concomitantly with radiotherapy and subsequently as monotherapy treatment. We report the case of a patient who developed toxic epidermal necrolysis (TEN) while she was being treated with chemoradiotherapy and several drugs. Cutaneous tests were performed with the drugs involved with negative result. Although the occurrence of TEN contraindicates suspected drug readministration, we based the decision to perform the controlled administration of temozolomide on the following reasons: (1) the poor prognosis of the underlying disease, (2) the lack of therapeutic alternatives, (3) the suspicion that other drugs taken by the patient simultaneously may be responsible (as anticonvulsants and trimethoprim sulfamethoxazole [TMP-SMX]), and (4) temozolomide was the first choice for treating the patient's disease. The administration of a cumulative dose of 60 mg of temozolomide caused a slight skin reaction. Given this result, we conducted controlled administration of other drugs involved. Dexamethasone, codeine, omeprazole and levetiracetam were well tolerated. However, TMP-SMX produced a similar reaction to that caused by temozolomide. In conclusion, we present the first case of TEN induced by temozolomide and TMP-SMX associated with cranial radiotherapy confirmed by controlled administration. Radiotherapy in combination with these drugs could have favored TEN, as some authors have postulated, but we cannot prove this.
Temozolomide is an oral alkylating agent indicated for the treatment of patients with glioblastoma multiforme concomitantly with radiotherapy and subsequently as monotherapy treatment.1 Toxic epidermal necrolysis (TEN) is an acute life-threatening mucocutaneous disorder with detachment of body surface area >30%. The majority of cases of TEN are the result of a hypersensitivity reaction to a drug.2 We report the case of a patient who developed TEN while she was being treated with chemoradiotherapy and several drugs.
A 37-year-old woman was being treated for a frontal glioblastoma multiforme with temozolomide (160 mg daily), cranial radiotherapy (60 Gy daily from Monday to Friday), levetiracetam, trimethoprim-sulfamethoxazole (TMP-SMX), omeprazole, codeine and dexamethasone. All drugs were started simultaneously. At 3-4 weeks of treatment, the patient started with eye symptoms (conjunctival hyperemia, epiphora, itching and discharge mucopurulent) that were attributed to radiotherapy by doctors in the Emergency Room. Four days later she added burning sensation and macular rash on her face, and in the next 24 hours erythematous violaceous macules appeared in cranial region, face, ears and neck with intense burning. Besides skin involvement, she had genital burning, but no malaise, fever or itching. The patient continued treatment and she was treated with methylprednisolone and dexchlorpheniramine on three occasions in the emergency services. One week later, she was evaluated in our clinics for worsening with dysphagia, malaise, bullous lesions on ears and face, and violaceous macules (not blanched on pressure) and confluent target-like lesions in the abdomen, proximal part of the upper extremities and legs, palms and soles. The patient had fever (37.5℃) and Nikolsky's sign was positive. The evaluation by ophthalmologist revealed crusty lid margins, tarsal and bulbar hyperemia, left inferior tarsus membranes, clear corneas without ulcers and transparent media with normal iris. Previous ocular symptoms had worsened and the patient also had ocular pain and photophobia.
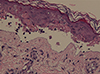
The patient was hospitalized with a SCORTEN (Severity of Illness Score for Toxic Epidermal Necrolysis) of 2.3 On admission, the blood count and coagulation studies were normal, biochemistry parameters were within their respective reference ranges except total cholesterol (308 mg/dL), and blood cultures proved negative. Radiotherapy and medication that the patient was taking were discontinued, and she was treated with supportive measures and intravenous methylprednisolone, antihistamines and ranitidine. Nevertheless, the blistering rash became widespread in about 24 hours with palmoplantar involvement and denudation of the skin over 30% of her body surface. In addition to previous treatment, she was treated with dexamethasone and erythromycin eye drops, mouthwashes with lidocaine, intravenous amoxicillin-clavulanate and subcutaneous enoxaparin. The patient gradually improved within 4 weeks, and the only sequel was just dry eye. The skin biopsy performed the day after hospital admission was consistent with TEN and showed keratinocyte necrosis and subepidermal blisters (Figure). Six weeks after the reaction, patch tests and intradermal tests were performed with negative result (Table 1).
Although the occurrence of TEN contraindicates suspected drug readministration, we based the decision to perform the controlled administration of temozolomide on the following reasons: (1) the poor prognosis of the underlying disease, (2) the lack of therapeutic alternatives, (3) the suspicion that other drugs taken by the patient simultaneously may be responsible (as anticonvulsants and trimethoprim sulfamethoxazole [TMP-SMX]), and (4) temozolomide was the first choice for treating the patient's disease.1 Ten weeks after cessation of radiotherapy, and once the patient signed the informed consent (July 20), we administered 20 mg of temozolomide orally and 30 minutes later 40 mg. Half an hour later the patient had burning sensation and redness in her face, anterior chest and arms with macules on her back. She improved in about 2 hours after administration of methylprednisolone and dexchlorpheniramine. The patient was kept in hospital and 6 hours later erythema and skin burning sensation reappeared and was controlled with the same treatment by one hour. Given this result, we conducted controlled administration of other drugs involved. Omeprazole (September 4), codeine (September 5), dexamethasone (September 27) and levetiracetam (November 29) were well tolerated. However, administration of a quarter of a tablet of TMP-SMX (160/800 mg) (October 20) produced at 30 minutes a similar reaction to that caused by temozolomide, but slighter, that disappeared within one hour after corticosteroids and antihistamines administration (there was no delayed reaction).
The main problem when a severe reaction occurs in a patient polymedicated is to identify the responsible drug. Unfortunately, there are no diagnostic tests useful in the case of TEN and in vivo tests must be made with caution. In our case, the serious situation of the patient motivated us to search for the causative drug by skin tests, but they were not useful (as in other publications4).
Hypersensitivity reactions (as erythema multiforme, erythroderma, urticaria, exantema, anaphylaxis, and angioedema) have been rarely described with temozolomide.1,4,5 In the literature, we have found one case of Stevens-Johnson syndrome (SJS) and TEN overlap in a patient treated with oral temozolomide, cranial radiotherapy, diclofenac and phenytoin,6 and other case of TEN in a patient treated with the same drugs excepting diclofenac.7 In the first case, the authors assumed that temozolomide was responsible for the reaction, since the patient did not discontinue phenytoin and diclofenac despite the reaction, and he improved.6 In the second case, both phenytoin and temozolomide could be responsible for the reaction, and the authors further pointed out the possibility that radiation can promote reactions with some drugs such as phenytoin and other anticonvulsants.7
Obtaining a positive result with temozolomide with mild reaction encouraged us to test the rest of the drugs, as all of them were necessary to improve the quality of life of the patient. However, we were surprised by the positivity of TMP-SMX, as it is not common to find multiple etiologies in TEN. Interestingly, there is a reported case of a patient with a history of allergy to TMP-SMX that presented an anaphylaxis reaction with temozolomide.4
In the literature, it is postulated that radiotherapy could enhance the ability of some drugs to produce TEN, overall anticonvulsivants and aminofostine.8 Several mechanisms have been proposed for the involvement of radiotherapy in the development of SJS and TEN. The addition of radiotherapy may cause an enzyme inhibition or deficiency, which in turn would prevent biotransformation of the reactive metabolites. As these accumulate, they may act as haptens and initiate a secondary immunologic response. On the other hand, radiotherapy could also modify the pool of T-suppressor lymphocytes.7 In our case, the reactions due to controlled administration of temozolomide and TMP-SMX without radiotherapy were earlier and milder than the first reaction. It has been described that upon readministration of the implicated drug, TEN may develop within hours,2 and the earlier the causative drug is withdrawn, the better the prognosis.3 In our case, we administered the drug fractionally and the patient had a mild and early reaction with sub-therapeutic doses. We immediately treated this reaction, which could prevent the progression of the reaction. There is no experience in the literature on controlled drug challenge when TEN is suspected, so we unknown if these milder reactions would be expected. In addition to that, we propose as a hypothesis that radiotherapy administered with the drugs involved could have influenced the severity of the reaction, but obviously we cannot prove this.
In conclusion, we present the first case of TEN induced by temozolomide and TMP-SMX associated with cranial radiotherapy confirmed by controlled administration. Radiotherapy in combination with these drugs could have favored TEN, as some authors have postulated.
Figures and Tables
Figure
Histology of TEN. The skin biopsy performed the day after hospital admission was consistent with TEN and showed keratinocyte necrosis and subepidermal blisters.
Table
Cutaneous tests with the drugs involved
References
1. European Medicines Agency (GB). Annex I: summary of product characteristics [Internet]. London: European Medicines Agency;2013. cited 2013 Dec 4. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001128/WC500079539.pdf.
2. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013; 69:173.e1–173.e13.
3. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013; 69:187.e1–187.e16.
4. Mónica RG, De Las Heras Gozalo M, de la Barrera EH, Dominguez JS. Successful desensitization with temozolomide. Ann Allergy Asthma Immunol. 2011; 106:541–542.
5. Alonso-Llamazares A, Vega-Castro A, Beitia-Mazuecos JM, Mateo-Borrega B, Cardenas-Contreras R. Rapid desensitization with temozolomide in patients with delayed maculopapular rash. J Investig Allergol Clin Immunol. 2012; 22:448–449.
6. Sarma N. Stevens-Johnson Syndrome and toxic epidermal necrolysis overlap due to oral temozolomide and cranial radiotherapy. Am J Clin Dermatol. 2009; 10:264–267.
7. Poletti ED, Muñoz Sandoval R, Guzmán Perera G, González Fisher RF, Márquez Díaz F. Melanoma metastásico, radioterapia holocraneal y dermatitis por aumento de la radiación. Dermatología Rev Mex. 2011; 55:388–394.
8. Vern-Gross TZ, Kowal-Vern A. Erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis syndrome in patients undergoing radiation therapy: a literature review. Am J Clin Oncol. 2014; 37:506–513.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download