Abstract
Eosinophils have been reported to modulate T cell responses. Previously, we reported that high-mobility group box 1 protein (HMGB1) played a key role in the pathogenesis of asthma. This study was conducted to test our hypothesis that eosinophils could modulate T cell responses via HMGB1 in the pathogenesis of asthma characterized by eosinophilic airway inflammation. We performed in vitro experiments using eosinophils, dendritic cells (DCs), and CD4+ T cells obtained from a murine model of asthma. The supernatant of the eosinophil culture was found to significantly increase the levels of interleukin (IL)-4 and IL-5 in the supernatant of CD4+ T cells co-cultured with DCs. HMGB1 levels increased in the supernatant of the eosinophil culture stimulated with IL-5. Anti-HMGB1 antibodies significantly attenuated increases of IL-4 and IL-5 levels in the supernatant of CD4+ T cells co-cultured with DCs that were induced by the supernatant of the eosinophil culture. In addition, anti-HMGB1 antibodies significantly attenuated the expressions of activation markers (CD44 and CD69) on CD4+ T cells. Our data suggest that eosinophils modulate CD4+ T cell responses via HMGB1 in the pathogenesis of asthma.
Eosinophils have long been recognized as terminal effector cells. However, recent reports have suggested that eosinophils might participate in initial stages of allergy development by modulating T-cell responses.1 Moreover, pulmonary eosinophils are required for the localized recruitment of T cells.2,3 High-mobility group box 1 protein (HMGB1) is a non-histone DNA-binding nuclear protein that act as a endogenous 'Danger signal' and trigger innate immune reactions.4 Previously, we reported that HMGB1 played a key role in the pathogenesis of asthma characterized by eosinophilic airway inflammation.5 HMGB1 is a well-characterized ligand of the receptor of advanced glycation end products (RAGE).6 Recently, it was reported that house dust mite (HDM) sensitization induced the release of HMGB1 from the airway epithelium and that the adoptive transfer of HDM-pulsed RAGE+/+ dendritic cells to RAGE-/- mice recapitulated the eosinophilic responses after HDM challenge.7 However, no report has evaluated the effect of HMGB1 on T cell functions in the eosinophilic airway inflammation. In this study we hypothesized that eosinophils could modulate T cell functions via HMGB1 in the pathogenesis of asthma. To test our hypothesis, we collected eosinophils, dendritic cells (DCs), and CD4+ T cells from a murine model of asthma. We evaluated the effect of eosinophils on the activation and cytokine release of CD4+ T cells co-cultured with DCs. We also assessed whether those effects were mediated via HMGB1.
All experimental procedures were performed with the approval from the Seoul National University Institutional Animal Care and Use Committee. Female BALB/c mice (6 weeks old) were purchased from Orient Bio (Sungnam, Korea). A murine model of asthma was generated after intraperitoneal sensitization with ovalbumin (OVA) plus alumhydroxide and a subsequent intranasal challenge with OVA alone. Detailed methods were described previously.5 Mice were sacrificed after the last challenge. Perfused lung tissues were chopped and incubated in 0.5% EDTA for 15 minutes at 37℃. Lymph nodes were removed from the drained region of each lung. To obtain single cell suspensions, chopped lung tissue and lymph nodes were ground onto a 0.7 µm strainer with complete RPMI-1640 (cRPMI-1640) cell culture media supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin/streptomycin. Erythrocytes were lysed with ammonium chloride solution (StemCell Technologies, Vancouver, Canada). Cell pellets were washed twice with cRPMI-1640 followed by centrifugation at 1,500 g for 10 minutes. Dendritic cells (DCs; here we defined the CD11b-CD11c+ cells as dendritic cells) were separated from total lung cells using anti-CD11b (FITC-CD11b) and anti-CD11c (PerCP-Cy5.5-CD11c) (eBioscience, San Diego, CA, USA). Labeled cells were analyzed with FACSCalibur and CellQuest software (BD Biosciences, San Jose, CA). CD4+ T cells were separated from lymph node cells using anti-CD4 microbeads (Miltenyi Biotec, Auburn, CA, USA). Positive selection was performed using an automatic magnetic cell-separation sorter (Auto MACS sorter, Miltenyi Biotec, Auburn, CA, USA). Mice blood was collected from heart punctures, diluted in RPMI-1640, layered on Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ, USA), and subjected to density gradient centrifugation. The buffy-coat containing lymphocytes and granulocytes was removed and washed twice in RPMI-1640 containing 2% FBS. Cell pellets were then subjected to brief hypotonic lysis in order to disrupt any contaminating red blood cells using ammonium chloride solution. Eosinophils were stained with FITC-conjugated CD16/32 and then subjected to anti-FITC microbeads (Miltenyi Biotec, Auburn, CA, USA).2,8 Stained cells were negatively separated using the automatic magnetic cell-separation sorter. Eosinophils were subsequently enriched by removing lymphocytes using the Auto MACS sorter. Briefly, B cells and T cells were removed by positive selection and then incubated with antibody-conjugated magnetic beads specific for CD45-R (B220) and CD90 (Thy 1.2), which bind to B and T cells, respectively. Cell purities were assessed by H&E staining and the purity standard was set to be >90%.
First, the levels of IL-4 and IL-5 in the supernatant of CD4+ T cells co-cultured with DCs were measured after their incubation with the supernatant (50 µL) of the eosinophil culture which was obtained after incubation with IL-5 (an eosinophil activator, 10 ng/mL) for 12 hours. Undetectable IL-5 in the supernatant of the eosinophil culture was confirmed before the supernatant was added to the co-culture of CD4+ T cells and DCs. Therefore, it is unlikely that IL-5 in the supernatant of the eosinophil culture affected IL-5 measurements in the supernatant of CD4+ T cells co-cultured with DCs. We evaluated whether the levels of HMGB1 increased in the supernatant of the eosinophil culture after stimulation with IL-5. We then measured the levels of IL-4 and IL-5 in the supernatant of CD4+ T cells co-cultured with DCs. Finally we evaluated the expression of CD44 and CD69 on CD4+ T cells after incubation with the supernatant (50 µL) of the eosinophil culture alone or plus anti-HMGB1 antibodies (2 µg/mL). CD44 and CD69 were reported to be T cell activation markers.9 Levels of cytokine and HMGB1 in the culture supernatants were determined by ELISA. Expressions of marker were detected by flow cytometry using Cell Quest software (BD, Mountain View, CA, USA).
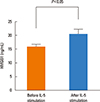
The levels of IL-4 and IL-5 in the supernatant of CD4+ T cells co-cultured with DCs significantly increased after incubation with the supernatant of the eosinophil culture (Fig. 1). We then observed that HMGB1 levels were significantly elevated in the supernatant of the eosinophil culture stimulated with IL-5 (Fig. 2). Increases in IL-4 and IL-5 levels in the supernatant of CD4+ T cells co-cultured with DCs and CD44 and CD69 expressions on CD4+ T cells after incubation with the supernatant of the eosinophil culture were significantly attenuated when anti-HMGB1 antibodies were added (Fig. 3 and 4).
The present study was conducted to test our hypothesis that eosinophils could modulate T cell responses via HMGB1 in the pathogenesis of asthma. We performed in vitro experiments using eosinophils, dendritic cells (DCs), and CD4+ T cells obtained from a murine model of asthma. Our results revealed that the supernatant of the eosinophil culture significantly increased the levels of IL-4 and IL-5 in the supernatant of CD4+ T cells co-cultured with DCs. In our study, HMGB1 levels increased in the supernatant of the eosinophil culture stimulated with IL-5, suggesting that HMGB1 might be secreted from the activated eosinophil. Our results also demonstrated that anti-HMGB1 antibodies significantly attenuated the increases of IL-4 and IL-5 levels in the supernatant of CD4+ T cells co-cultured with DCs that were induced by the supernatant of the eosinophil culture. HMGB1 antibodies also significantly reduced the expressions of activation markers (CD44 and CD69) on CD4+ T cell. It was reported that intratracheal transfer of eosinophil into IL-5 null mice exposed to antigen resulted in the restoration of asthma phenotypes, strongly suggesting CD4+ T cell-mediated inflammatory signals as well as signals derived from eosinophils cooperatively contributed to the development of asthma.10 Taken together, it is possible that HMGB1 may be one of the important mediators released from eosinophils.
Previous studies reported that DC-conditioned medium containing HMGB1 polarized CD4+ T cells toward the Th1 phenotype,11,12 which is different from our findings. The discrepancy could be due to the fact that previous studies used naïve T cells whereas our study used CD4+ T cells obtained from a murine model of asthma. Those CD4+ T cells might have already been primed under the Th2-deviating microenvironment. The effects of HMGB1 on naïve CD4+ T cells might be different from those on Th2 primed CD4+ T cells. For example, the cooperative role of the CD4+ T cell-mediated inflammatory signals and signals derived from eosinophils were only seen in OVA-treated IL-5-/- mice, but not in naive IL-5-/- mice.10 Detectable levels of baseline IL-4 and IL-5 in the supernatant of CD4+ T cells co-cultured with DCs (as seen in the 'Culture media only' group in Fig. 1) and detectable levels of baseline HMGB1 in the supernatant of the eosinophil culture (as seen in the 'Before IL-5 stimulation' group in Fig. 2) could be explained by the fact that all cells were derived from an established murine model of asthma. Therefore, effects of HMGB1 on CD4+ T cells may differ depending on the functional states of CD4+ T cells.
It was reported that the cDNA sequence of a conserved lymphokine elements-0 (CLE0) binding protein (CLEBP-1) was almost identical to the cDNA sequences of HMGB1.13 CLEBP-1 binds to CLE0 in the promoter region of IL-5 which is essential for the expression of IL-5.14 A murine model for spontaneous osteoarthritis exhibited not only elevated levels of HMGB1 in hyperplastic synovium but also elevated levels of IL-5 in serum.15 In addition, the HMGB1 protein and messenger RNA levels of HMGB1 and IL-5 were significantly higher in eosinophilic chronic rhinosinusitis with nasal polyps than those from controls or non-eosinophilic chronic rhinosinusitis with nasal polyps.16 Taken together, these findings suggest that HMGB1 might be closely linked to IL-5 production.
In conclusion, our in vitro data revealed that eosinophils modulated CD4+ T cell responses via HMGB1 in the pathogenesis of asthma characterized by eosinophilic airway inflammation, although in vivo experiments are needed to confirm our findings. Along with our previous report, we conclude that HMGB1 might be a therapeutic target in the treatment of asthma.
Figures and Tables
Fig. 1
IL-4 and IL-5 levels in the supernatant of CD4+ T cells co-cultured with dendritic cells after incubation with the supernatant of the eosinophil culture.

Fig. 3
IL-4 and IL-5 levels in the supernatant of CD4+ T cells co-cultured with dendritic cells after incubation with the supernatant of the eosinophil culture alone or plus anti-HMGB1 antibodies.
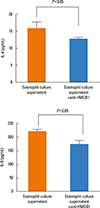
Fig. 4
CD44 and CD69 expressions on the CD4+ T cells after incubation with the eosinophil culture supernatant alone or with the eosinophil culture supernatant plus anti-HMGB1 antibodies. Relative expression was represented as a ratio compared to the mean fluorescence intensity of each molecule treated with media only.

References
1. Walsh ER, August A. Eosinophils and allergic airway disease: there is more to the story. Trends Immunol. 2010; 31:39–44.
2. Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008; 205:699–710.
3. Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008; 205:1285–1292.
4. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005; 5:331–342.
5. Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, et al. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012; 42:958–965.
6. Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol. 2012; 33:633–640.
7. Ullah MA, Loh Z, Gan WJ, Zhang V, Yang H, Li JH, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014; 134:440–450.e3.
8. Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, et al. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002; 71:1033–1041.
9. Liu L, Inoue H, Nakayama H, Kanno R, Kanno M. The endogenous danger signal uric acid augments contact hypersensitivity responses in mice. Pathobiology. 2007; 74:177–185.
10. Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003; 170:3296–3305.
11. Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007; 220:35–46.
12. Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004; 173:307–313.
13. Marrugo J, Marsh DG, Ghosh B. The conserved lymphokine element-0 in the IL5 promoter binds to a high mobility group-1 protein. Mol Immunol. 1996; 33:1119–1125.
14. Masuda ES, Tokumitsu H, Tsuboi A, Shlomai J, Hung P, Arai K, et al. The granulocyte-macrophage colony-stimulating factor promoter cis-acting element CLE0 mediates induction signals in T cells and is recognized by factors related to AP1 and NFAT. Mol Cell Biol. 1993; 13:7399–7407.
15. Kyostio-Moore S, Nambiar B, Hutto E, Ewing PJ, Piraino S, Berthelette P, et al. STR/ort mice, a model for spontaneous osteoarthritis, exhibit elevated levels of both local and systemic inflammatory markers. Comp Med. 2011; 61:346–355.
16. Chen D, Mao M, Bellussi LM, Passali D, Chen L. Increase of high mobility group box chromosomal protein 1 in eosinophilic chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2014; 4:453–462.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download