Abstract
Purpose
The complex interplay between environmental and genetic factors plays an important role in the development of asthma. Several studies have yielded conflicting results regarding the 2 asthma-related risk factors: antibiotic usage during infancy and/or a history of bronchiolitis during early life and the development of asthma. In addition to these risk factors, we also explored the effects of Toll-like receptor 4 (TLR4) polymorphism on the development of childhood asthma.
Methods
This cross-sectional study involved 7,389 middle school students who were from 8 areas of Seoul, Korea, and completed the International Study of Asthma and Allergies in Childhood questionnaire. The TLR4 polymorphism rs1927911 was genotyped in 1,395 middle school students from two areas using the TaqMan assay.
Results
Bronchiolitis in the first 2 years of life, antibiotic exposure during the first year of life, and parental history of asthma were independent risk factors for the development of asthma. When combined, antibiotic use and a history of bronchiolitis increased the risk of asthma (adjusted odds ratio [aOR]: 4.64, 95% confidence interval [CI]: 3.09-6.97, P value for interaction=0.02). In subjects with CC genotype of TLR4, antibiotic exposure and a history of bronchiolitis during infancy, the risk of asthma was increased, compared to subjects without these risk factors (aOR: 5.72, 95% CI: 1.74-18.87).
Conclusions
Early-life antibiotic exposures and a history of bronchiolitis are risk factors for asthma in young adolescents. Polymorphisms of TLR4 modified the influence of these environmental factors. Reducing antibiotic exposure and preventing bronchiolitis during infancy may prevent the development of asthma, especially in genetically susceptible subjects.
The prevalence of asthma has increased markedly worldwide during the second half of the 20th century.1 This increase has resulted in extensive investigation of risk factors responsible for asthma development. Based on the hygiene hypothesis, infections and exposure to microorganisms early in life protect against the development of allergic diseases later.2 Recently, this hypothesis was revisited and microbial hypothesis addresses the importance of gut microbiota, because a normal commensal gut microbiota promotes intestinal cell homeostasis and the appropriate development of the immune system.3
Antibiotic exposure has been known to alter gut microbiota and metabolism,4,5 and several studies have demonstrated that antibiotic use in infancy is associated with the subsequent development of asthma.5,6,7,8,9,10 The combination of the hygiene hypothesis and the microbial hypothesis may, at least in part, explain the impaired barrier function of the intestine and the Th1 immune response often associated with the development of asthma.11,12,13 However, the underlying mechanism has not been fully elucidated.
A history of respiratory tract infection during early life is another risk factor involved in the development of asthma.14 One study has shown that infants hospitalized for respiratory syncytial virus (RSV) bronchiolitis have an increased risk (2- to 3-fold) of developing asthma later in life.15 Further, another study has demonstrated that rhinovirus-induced wheezing during the first 3 years of life were associated with a nearly 10-fold increase in asthma development by 6 years of age.16
Furthermore, the development of asthma is influenced by polymorphisms in innate immune-related genes.17 For instance, genes involved in the pathophysiology of asthma such as allergic inflammation and regulation of immunoglobulin E (IgE) are influenced by genetic polymorphisms of Toll like receptor 4 (TLR4) that encode pattern recognition receptors and extracellular receptors.18,19,20 Moreover, polymorphisms in TLR4 have been associated with atopic sensitization, atopy severity,21 and atopic asthma.20,22 These associations reflect the important role of innate immunity genes in the development of allergic diseases, including asthma, via the interactions between host and microbe.23
In the present study, we have investigated the risk factors involved in the development of asthma during early life and their interactions. Specifically, we investigated whether antibiotic exposure in the first year of life and a history of physician-diagnosed bronchiolitis in the first 2 years of life were associated with an increased risk of childhood asthma. In addition, we evaluated whether a polymorphism in TLR4 (rs1927911) modulates the impact of these environmental factors based on the fact that these polymorphisms were shown to be associated with asthma in previous studies.21,22
For this study, middle school students from 8 schools in 5 areas of Seoul (downtown, northeastern, northwestern, southeastern, and southwestern), Korea, were randomly recruited in 2008 (n=4,094) and 2011 (n=3,295). Since these two groups did not differ significantly in terms of baseline characteristics, each data was merged to maximize the power of the analysis. Thus, total 7,389 students participated in the present study.
A modified version of the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaire24 was completed by all study participants. The questionnaire consisted of 3 sections with questions asking about (1) general characteristics including sex, date of birth, height, and weight; (2) the history of asthma- and wheezing-related symptoms; and (3) environmental factors that are associated with allergic diseases. This study was approved by the Institutional Review Board of Asan Medical Center, Ulsan University, Seoul, Korea, and written informed consent was obtained from the parents or guardians of all participants.
The prevalence of asthma was determined by the responses to the following questions: (1) "Have you ever had wheezing or whistling in the chest?" (wheeze, ever), (2) "Have you had wheezing or whistling in the chest in the last 12 months?" (wheeze in the last 12 months), (3) "Have you ever been diagnosed with asthma?" (asthma diagnosis, ever), and (4) "Have you been treated for asthma in the last 12 months?" (asthma treatment in the last 12 months). The asthma diagnosis was determined by the question "Have you ever been diagnosed with asthma?".
Information on antibiotic exposure in the first year of life was determined using a questionnaire. A history of bronchiolitis in the first 2 years of life was evaluated in the question "Has your child been diagnosed with bronchiolitis by a physician during the first 2 years of life?". The parental asthma history was assessed by the question "Have you ever been diagnosed with asthma by a doctor?" in the parent's questionnaire.
For our genotyping analysis, we focused on the 1,395 middle-school students from two schools (one in 2008 and the other in 2011). They were genotyped for the TLR4 rs1927911 polymorphism from their blood by using TaqMan fluorogenic 5'-nuclease assays (ABI, Foster City, CA, USA). All polymerase chain reactions were performed in 384-well plates using a 384-well Veriti thermal cycler (ABI, Foster City, CA, USA). Endpoint fluorescence readings were recorded with an ABI PRISM 7900 HT Sequence Detection System (ABI, Foster City, CA, USA). The frequency of the polymorphism in TLR4 (rs1927911) was in Hardy-Weinberg equilibrium (P>0.1). Duplicate samples and negative controls were included to ensure accuracy.
Statistical analyses were performed using the SPSS statistical software, version 21.0 (SPSS Inc, Cary, NC, USA). Odds ratios (ORs) and 95% confidence intervals (CI) were calculated to evaluate risk factors of the development of asthma by logistic regression analysis. In the multivariate analysis, adjustments were made on the basis of patient characteristics (age, sex, body mass index [BMI], school area, and household income), a genetic factor (parental history of allergic diseases), a socioeconomic factor (household income), and an environmental factor (environmental tobacco smoke). A P value of less than 0.05 was considered statistically significant.
The 7,389 middle school students (mean age±standard deviation, 13.90±0.89 years) were enrolled in this study. Table 1 displays the characteristics of the merged cohort from both 2008 and 2011. The prevalence of parental asthma history was 4.20%, while 63.26% had a history of passive smoking. There were no significant differences between children with and without information on the asthma diagnosis except a parental history of allergic diseases, household income, school area, passive smoking, and use of antibiotics during infancy (E-Table 1).
In the merged cohort, the lifetime prevalence of "wheeze, ever" was 14.27%, and the 12-month prevalence of wheeze was 7.52% (Table 2). The lifetime prevalence of asthma diagnosis was 7.77% and the prevalence of asthma treatment in the last 12 months was 1.74%.
After adjusting for age, sex, BMI, a parental history of allergic diseases, school area, and household income, independent risk factors for developing asthma were a history of bronchiolitis in the first 2 years of life (adjusted odd ratios [aOR]: 3.27, 95% CI: 2.32-4.60), antibiotic exposure for more than 3 days in the first year of life (aOR: 1.94, 95% CI: 1.49-2.53), and a parental history of asthma (aOR: 3.18, 95% CI 2.04-4.95; Table 3). The risk for developing asthma increased as the levels of antibiotic exposure rose (E-Table 2). Females were protected against the development of asthma in a consistent manner (aOR: 0.62, 95% CI: 0.47-0.82), whereas a high BMI was a risk factor for asthma only after adjusting for confounding factors (aOR: 1.05, 95% CI: 1.01-1.10). In addition, the presence of an older sibling was protective against asthma development (aOR: 0.75, 95% CI: 0.58-0.98).
Since two factors (a history of physician-diagnosed bronchiolitis in the first 2 years of life and antibiotic exposure for more than 3 days in the first year of life) were found to be independent risk factors for asthma, we assessed the combined effect of these 2 factors on the development of asthma (Table 4). The aOR was lower for participants who only had a history of antibiotic exposure (aOR: 1.56, 95% CI: 1.16-2.11) or a history of physician-diagnosed bronchiolitis (aOR: 2.16, 95% CI: 1.11-4.22). The combination of the 2 risk factors was found to be associated with an increased risk for developing asthma (aOR: 4.64, 95% CI: 3.09-6.97, P value for interaction=0.02).
On its own, the CC genotype of TLR4 rs1927911 was associated with an increased risk for the development of asthma (aOR: 2.21, 95% CI: 1.19-4.09; Table 3). This risk was further increased in infants with this TLR4 (rs1927911) polymorphism when they had a history of antibiotic exposure during the first year of life or a history of bronchiolitis during the first 2 years of life, compared to infants with CT+TT genotype and no history of antibiotics exposure or bronchiolitis history during early life (aOR: 3.46, 95% CI: 1.71-7.03, P value for interaction=0.14; aOR: 5.25, 95% CI: 2.02-13.67, P value for interaction=0.15, respectively; Table 4).
When we stratified the risk of asthma development according to the number of risk factors including antibiotic usage and bronchiolitis history during early infancy, and the CC genotype of TLR4 (rs1927911), we found that the risk of developing asthma increased with an increase in the number of the risk factors (Table 5). Compared to infants with no risk factors, the risk of developing asthma was increased in infants with three risk factors (aOR: 9.42, 95% CI: 3.08-28.82).
In addition, we stratified the risk of asthma according to antibiotic usage and bronchiolitis during infancy in each genotype of TLR4 (rs1927911). Subjects with the CC genotype of TLR4 were at a significantly increased risk of developing asthma with an aOR of 5.72, when they had both antibiotic usage and bronchiolitis during infancy (95% CI: 1.74-18.87; Figure).
In this study, we have shown that a history of physician-diagnosed bronchiolitis in the first 2 years of life, antibiotic exposure for more than 3 days in the first year of life, and a parental history of asthma are independent risk factors for the development of asthma in middle school students. The simultaneous presence of 2 of these three independent risk factors appeared to have an accumulative effect, whereby each additional risk factor increased the risk of developing asthma. This study has also provided evidence that the development of asthma is affected by a complex interplay between genetic and environmental factors: individuals with the CC genotype of TLR4 (rs1927911) were more likely to develop asthma when this genetic factor was combined with antibiotic exposure in the first year of life and/or a history of physician-diagnosed bronchiolitis in the first 2 years of life. To our knowledge, this is the first study on the combined effects of bronchiolitis at a young age, antibiotic exposure during infancy, and genetic variants in asthma-related genes such as TLR4 on the development of asthma.
Notably, the prevalence of asthma diagnosis in middle school students from Seoul, Korea was 7.8%. This was similar to the prevalence that was previously reported by a nationwide cross-sectional questionnaire study in school-aged (8-11 years old) children.25 Since the prevalence of asthma appears to be stable,26 we combined the data from both the 2008 and 2011 surveys to improve the power of our analyses examining the gene-environment interactions in the development of asthma. This merging of data was possible as both surveys were conducted in similar age groups (13-15 years old) in the same city using the same study methods by the same researchers. Moreover, the results of the present study were consistent with those of previous studies with respect to the risk factors for asthma.27 As a result of the merging of the data, the sample size was relatively sufficient to assess how antibiotic exposure in the first year of life and history of physician-diagnosed bronchiolitis in the first 2 years of life affect the subsequent development of asthma in genetically predisposed school-aged children.
Further, the polymorphism of TLR4 (rs1927911) was selected as a candidate gene because several studies, including our own, have shown that polymorphisms of this gene are associated with innate immune and immunoregulatory mechanisms that participate in the development of asthma.5,22,28 A study on the association between TLR4 polymorphisms and asthma in Swedish children showed that the TLR4 Asp299Gly polymorphism is associated with a higher prevalence of asthma in school-aged children (aOR: 4.5, 95% CI: 1.1-17.4).20 In another study, the TLR4 Asp299Gly and Thr399Ile polymorphisms were associated with hyporesponsiveness to inhaled lipopolysaccharide.29 However, in the present study, significant associations between the TLR4 (rs1927911) polymorphism, serum eosinophil counts, total serum IgE levels, and pulmonary function were not observed (data not shown).
In this study, CC genotype of TLR4 (rs1927911) was associated with an increased risk for childhood asthma. However, children with CT+TT genotype showed a significantly increased risk for asthma when they had a history of bronchiolitis and no history of antibiotic exposure. This discrepancy may be associated with the small sample size who met the criteria in the analysis of this combination or adjustment factors used in the analysis.
The present study revealed a strong positive association between antibiotic exposure in early life and the subsequent development of asthma. A recent review summarized the associations between antibiotic exposure in newborns and altered infant microbiota profiles, including a long-term reduction in microbiota diversity.30 Antibiotic-induced alterations in commensal microflora are believed to interfere with the postnatal maturation of the immune system.31 A supporting study performed using an experimental animal model has shown that treating mice with antibiotics disturbs their intestinal microflora and prevents postnatal Th1 cell maturation, thereby resulting in Th2-polarized immune deviation.32 These deviations in immune system development have been associated with the development of asthma, suggesting a link between early antibiotic exposure, immune function, and asthma.
Respiratory viral infections during critical developmental periods can interfere with normal lung growth, which has been shown to increase the risk of asthma.14 Further, the innate immune response to a fusion protein from RSV, one of the most common respiratory infectious pathogens in early life, is mediated by TLR4 and CD14.14,33 Furthermore, RSV infection sensitizes the airway epithelia to other pathogenic environmental factors, which can further induce signaling via TLR4 in a CD14-dependent fashion.34 Although the role of other viral pathogens in the development of asthma has not been fully explored, the conserved nature of the TLRs and their roles in innate immunity suggest that other infectious pathogens may also activate the innate immune response via the Toll signaling pathway.
The association between antibiotic exposure and bronchiolitis during infancy on the increased risk for developing asthma might be partially due to antibiotic use during bronchiolitis and early damage to the airway as a consequence of viral infection, including bronchiolitis during infancy.35 Airway damage increases vulnerability to respiratory viral infections and has been linked to exposure to harmful environmental factors that are associated with the increased risk of asthma. In addition, viral infection can enhance allergic sensitization and further increase the risk of asthma development in later life.36
Microbial exposure during early life is a major trigger of immune response changes during the development of immune system. The present epidemiological study showed associations between history of physician-diagnosed bronchiolitis in the first 2 years of life, antibiotic exposure in the first year of life, and genetic susceptibility to asthma. These associations may be explained by the fact that viral respiratory infections such as bronchiolitis sensitize the airway epithelia to the subsequent pathogenic environmental exposure via TLR4 signaling, while antibiotic exposure in the first year of life associates with changes in the composition of the gut microbiota and the development of TLR4-mediated innate antiviral immunity. These complex interactions may work together to modulate the risk of asthma.
The present study had several limitations. First, as it was a cross-sectional study, a cause-effect relationship cannot be confirmed. However, previous prospective cohort studies that adequately adjusted for these confounding factors indicated that they only play a small role in the relationship between antibiotic exposure during infancy and the subsequent development of asthma.7,10 In order to fully understand the link between the independent risk factors examined here and asthma development, additional large-scale prospective studies are needed. Second, although data from two recruitment periods were merged, the relatively low prevalence of asthma and small sample size are not sufficient to prove the complex interplay between genetic and environmental factors. Third, since antibiotics use and a history of physician-diagnosed bronchiolitis was assessed by using a questionnaire, recall bias may exist. Fourth, since asthma diagnosis was assessed using a questionnaire, there might be some possible discrepancies with the actual asthma diagnosis. Fifth, as this study is an epidemiologic study using a questionnaire, there was a large proportion of missing data especially in important variables such as a parental history of allergic diseases. Finally, although many genes have been found to be associated with the increased susceptibility to asthma, the present study only examined a single polymorphism in TLR4 (rs1927911) that we have previously shown to be associated with allergic diseases.22 Additional studies that elucidate the interactions between other polymorphisms in candidate genes and the development of asthma are required.
Genetic variants of TLR4 (rs1927911) appears to modify the combined effects of infant antibiotic exposure in the first year of life and a history of physician-diagnosed bronchiolitis in the first 2 years of life on the development of asthma. We suspect that reducing antibiotic exposure and preventing bronchiolitis during infancy may prevent the development of asthma, especially in genetically susceptible children. To confirm the results of this study and to determine the underlying mechanisms, larger-scale longitudinal studies are required.
Figures and Tables
Figure
Combined effect of TLR4 (rs1927911) genotypes with antibiotics exposure before 1 year of life and bronchiolitis in the first 2 years of life in middle school students for asthma diagnosis. Data were analyzed by using logistic regression and adjusted for age, gender, body mass index, a parental history of any allergic diseases, exposure to tobacco smoking, and household income. *P value <0.05.

Table 1
Baseline characteristics of the subjects
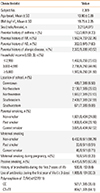
Table 2
Prevalence of asthma in middle school students from Seoul

Table 3
Risk factors of asthma diagnosis among middle school students from Seoul

Table 4
Combined effect of antibiotic exposure before 1 year of age, bronchiolitis during the first 2 years of life, and the TLR4 rs1927911 polymorphism on the risk of asthma development among middle school students

Table 5
Risk of asthma diagnosis according to number of risk factors including antibiotic usage, a history of bronchiolitis during infancy, and the CC genotype of TLR4 rs1927911

*Risk factors include a history of antibiotics usage during the first year of life, a history of bronchiolitis during the first two years of life, and CC genotype of TLR4 (rs1927911); †Adjusted by age, gender, body mass index, a parental history of any allergic diseases, exposure to tobacco smoking, and household income.
aOR, adjusted odds ratio; CI, confidence interval.
ACKNOWLEDGMENTS
The authors thank the study participants, their parents, and school teachers, as well as the ISAAC epidemiological survey committee of the Korean Academy of Pediatric Allergy and Respiratory Disease.
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A092076).
References
1. Martinez FD, Vercelli D. Asthma. Lancet. 2013; 382:1360–1372.
2. Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000; 55:Suppl 1. S2–S10.
3. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989; 299:1259–1260.
4. Kim HB, Ahn KM, Kim KW, Shin YH, Yu J, Seo JH, et al. Cord blood cellular proliferative response as a predictive factor for atopic dermatitis at 12 months. J Korean Med Sci. 2012; 27:1320–1326.
5. Ahn KM, Lee MS, Hong SJ, Lim DH, Ahn YM, Lee HR, et al. Fever, use of antibiotics, and acute gastroenteritis during infancy as risk factors for the development of asthma in Korean school-age children. J Asthma. 2005; 42:745–750.
6. Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir J. 2011; 38:295–302.
7. Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009; 123:1003–1010.
8. Wickens K, Pearce N, Crane J, Beasley R. Antibiotic use in early childhood and the development of asthma. Clin Exp Allergy. 1999; 29:766–771.
9. Celedón JC, Fuhlbrigge A, Rifas-Shiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy. 2004; 34:1011–1016.
10. Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007; 131:1753–1759.
11. Levy J. The effects of antibiotic use on gastrointestinal function. Am J Gastroenterol. 2000; 95:S8–S10.
12. Brismar B, Edlund C, Nord CE. Impact of cefpodoxime proxetil and amoxicillin on the normal oral and intestinal microflora. Eur J Clin Microbiol Infect Dis. 1993; 12:714–719.
13. Russell SL, Finlay BB. The impact of gut microbes in allergic diseases. Curr Opin Gastroenterol. 2012; 28:563–569.
14. Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012; 18:726–735.
15. Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009; 9:73–78.
16. Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008; 178:667–672.
17. Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006; 7:95–100.
18. Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism* in the 5' flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999; 20:976–983.
19. Werner M, Topp R, Wimmer K, Richter K, Bischof W, Wjst M, et al. TLR4 gene variants modify endotoxin effects on asthma. J Allergy Clin Immunol. 2003; 112:323–330.
20. Fagerås Böttcher M, Hmani-Aifa M, Lindström A, Jenmalm MC, Mai XM, Nilsson L, et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J Allergy Clin Immunol. 2004; 114:561–567.
21. Yang IA, Barton SJ, Rorke S, Cakebread JA, Keith TP, Clough JB, et al. Toll-like receptor 4 polymorphism and severity of atopy in asthmatics. Genes Immun. 2004; 5:41–45.
22. Choi WA, Kang MJ, Kim YJ, Seo JH, Kim HY, Kwon JW, et al. Gene-gene interactions between candidate gene polymorphisms are associated with total IgE levels in Korean children with asthma. J Asthma. 2012; 49:243–252.
23. Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008; 8:169–182.
24. Hong SJ, Lee MS, Sohn MH, Shim JY, Han YS, Park KS, et al. Self-reported prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995-2000. Clin Exp Allergy. 2004; 34:1556–1562.
25. Suh M, Kim HH, Sohn MH, Kim KE, Kim C, Shin DC. Prevalence of allergic diseases among Korean school-age children: a nationwide cross-sectional questionnaire study. J Korean Med Sci. 2011; 26:332–338.
26. Anderson HR, Ruggles R, Strachan DP, Austin JB, Burr M, Jeffs D, et al. Trends in prevalence of symptoms of asthma, hay fever, and eczema in 12-14 year olds in the British Isles, 1995-2002: questionnaire survey. BMJ. 2004; 328:1052–1053.
27. Hong SJ, Kim SW, Oh JW, Rah YH, Ahn YM, Kim KE, et al. The validity of the ISAAC written questionnaire and the ISAAC video questionnaire (AVQ 3.0) for predicting asthma associated with bronchial hyperreactivity in a group of 13-14 year old Korean schoolchildren. J Korean Med Sci. 2003; 18:48–52.
28. Jung YH, Seo JH, Kim HY, Kwon JW, Kim BJ, Kim HB, et al. The relationship between asthma and bronchiolitis is modified by TLR4, CD14, and IL-13 polymorphisms. Pediatr Pulmonol. Forthcoming 2013.
29. Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000; 25:187–191.
30. Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011; 6:209–240.
31. Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012; 13:440–447.
32. Oyama N, Sudo N, Sogawa H, Kubo C. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol. 2001; 107:153–159.
33. Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000; 1:398–401.
34. Krishnan S, Halonen M, Welliver RC. Innate immune responses in respiratory syncytial virus infections. Viral Immunol. 2004; 17:220–233.
35. Martinez FD. Viral infections and the development of asthma. Am J Respir Crit Care Med. 1995; 151:1644–1647.
36. Schwarze J, Gelfand EW. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur Respir J. 2002; 19:341–349.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download