This article has been corrected. See "Erratum: In Vitro Evaluation of Allergen Potencies of Commercial House Dust Mite Sublingual Immunotherapy Reagents" in Volume 9 on page 187.
Abstract
Purpose
The clinical efficacy of allergen-immunotherapy is known to be dose dependent. However, optimal maintenance dosage has not yet been determined for sublingual immunotherapy (SLIT). Furthermore, since companies adopt their own units for expression of allergenicity, the allergen concentrations of individual reagents cannot be compared easily. We sought to measure and compare the allergenicities of 3 commercially available house dust mite (HDM) SLIT regents and a subcutaneous immunotherapy reagent.
Methods
We measured the HDM allergenic potency of the maintenance dosages of three SLIT reagents: Staloral® (300 index of reactivity [IR] /mL, recommended maintenance dosage [MD]: 120 IR), SLITone® (1,000 standard therapeutic unit [STU]/mL, recommended MD: 200 STU), Wolwopharma® (100 µg/mL, recommended MD: 20 µg), and subcutaneous immunotherapy regents of Hollister-Stier (10,000 allergy unit [AU] /mL). The allergenic potency was assessed by measuring the total protein concentrations, mite group 1 and 2 allergens using 2-site ELISA, and an inhibition test against IgE specific to Dermatophagoides farinae and Dermatophagoides pteronyssinus.
Results
The protein content of the Wolwopharma® reagent was 1.5-261.4 times higher than that of the other 2 SLIT reagents. The concentration of group 1 major allergens in Staloral® (132.03 µg/mL) was 33- to 44.5-fold higher than in SLITone® (4.00 µg/mL) and Wolwopharma® (2.97 µg/mL). The concentration of group 2 major allergen was also 8.9- to 10.5-fold higher in Staloral® (15.7 µg/mL) than in SLITone® (1.8 µg/mL) or Wolwopharma® (1.5 µg/mL). An ELISA inhibition study against HDM-specific IgE showed that the allergen potency of Staloral® reagent is 8.5-fold and 21-fold higher than that of SLITone® or Wolwopharma®, respectively. The differences between the maintenance dosages are further exaggerated by the differences in the recommended volumes of SLIT reagents.
Sublingual immunotherapy (SLIT) has been recognized as a safe and effective treatment for various respiratory allergic diseases.1,2,3 It is widely prescribed in European, South American, and Southeast Asian countries, including Korea4 and China. Safety is considered an advantage of SLIT over subcutaneous immunotherapy (SCIT). To date, only 11 anaphylaxis cases caused by SLIT have been reported.5 House dust mite (HDM)-induced respiratory allergy is a good candidate for SLIT. Meta-analysis showed that SLIT is more effective at treating HDM respiratory allergy than hay fever.1 As the efficacy of SCIT immunotherapy is dose-dependent,6,7,8 it is quite plausible that the efficacy of SLIT is also dose-dependent. In fact, several researchers have shown that the efficacy of SLIT administration by tablets in treating grass pollen allergic rhinoconjunctivitis patients is dose dependent.9,10 Reflecting these results, a position paper from The American Academy of Allergy Asthma Immunology and American College of Allergy Asthma Immunology,7 as well as a guideline from the Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update,11 suggested that high-dose SLIT may be effective for the treatment of respiratory allergic diseases. However, they do not provide any detailed information about the optimal maintenance dose. Thus, the optimal maintenance dose of SLIT is still controversial and needs to be determined.3,7,9
The absence of consensus regarding the maintenance dose for SLIT has result in considerable discrepancies in the allergen potencies of commercially available SLIT reagents. HDM is the ubiquitous and most important indoor allergen in warm climate countries, and several studies have suggested that the allergenic potencies of commercially available HDM SLIT reagents are about 10- to 20-fold different according to their manufacturers.12,13 The fact that companies use their own house units for allergen potency makes it impossible to directly compare the allergen potencies of different SLIT reagents by simply reviewing the manufacturers' instruction leaflets.14,15 Accordingly, knowing the allergen potencies of commercial SLIT reagents may be highly useful for practicing allergists.
In this study, we compared the allergen potencies of HDM SLIT reagents from European and Chinese manufacturers using the ELISA inhibition test, immunoCAP inhibition tests, and two-site ELISA kits for measuring concentrations of the major HDM allergens in the SLIT reagents.
In this study, 3 HDM SLIT reagents were evaluated: Staloral® (Stallergen, Antony, France), SLITone® (AlkAbello, Madrid, Spain), and Wolwopharma® (Shanghai, China). For comparison, SCIT reagents Dermatophagoides farinae (10,000 allergy unit [AU]/mL), Dermatophagoides pteronyssinus (10,000 AU/mL), and their mixture (D. farinae 15,000 AU/mL and D. pteronyssinus 15,000 AU/mL) manufactured by Hollister-Stier (Spokane, WA, USA) were also evaluated. The Hollister-Stier reagents contain 50% (v/v) glycerin or 0.4% phenol as a preservative, 0.5% sodium chloride, and 0.275% sodium bicarbonate, in addition to HDM. Staloral® contains sodium chloride (0.059 g), glycerol (0.58 g), purified water, and 300 index of reactivity (IR) HDM in 1 mL. SLITone® contains sodium chloride (5 mg), glycerin (0.5 mL), sodium phosphate monobasic (5.95 mg), sodium phosphate dibasic (5.2 mg), sodium hydroxide, hydrochloric acid, and water in 1 mL of 1,000 standard therapeutic unit (STU). Wolwopharma® contains D. farinae and sodium chloride. Their allergen potencies, recommended administration volumes, and maintenance dosages are described in Table 1.
We measured group 1 and group 2 major allergens of HDM using two-site ELISA kits (Indoor Biotechnologies, Cardiff, UK) according to manufacturer's recommendation. It can differentiate the group 1 major allergens of D. pteronyssinus and. D. farinae, but it cannot differentiate the group 2 major allergen of D. pteronyssinus and D. farinae. The detection limits of Der p 1, Der f 1, and group 2 major allergens were 0.5 ng, 0.5 ng, and 0.2 ng, respectively.
For the ELISA inhibition test, pooled sera from 5 Korean allergic rhinitis patients sensitized to HDM were used. First, ELISA plate wells were coated with 10 µg/mL of D. pteronyssinus or D. farinae whole-body extract (Hollister-Stier) in 50 µL of bicarbonate buffer (pH 9.6) overnight. Then, 50 µL of serum samples (1:4-diluted in 1% bovine serum albumin in phosphate buffered saline, pH 7.4), which were pre-incubated with various quantities of inhibitor (threefold serially diluted from 30 µg to 137.17 ng) were added to each well and incubated for 1 hour. Subsequently, IgE antibodies were detected using biotinylated goat anti-human IgE (Vector, Burlingame, CA, USA) and streptavidin-peroxidase (Sigma-Aldrich, St. Louis, MO, USA). The color was developed with 3,3',5,5'-tetramethylbenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA). The absorbance at 450 nm was measured after addition of 0.5 M H2SO4 to stop color development.
For the ImmunoCAP inhibition assay, sera from 30 Korean allergic rhinitis patients sensitized to HDM were used. Fifty microliters of each serum was incubated for 2 hours with various concentrations of inhibitors at room temperature. Each inhibitor was diluted fivefold serially from 100% v/v to 0.0064% v/v using 1% bovine serum albumin in phosphate-buffered saline. IgE antibodies against D. farinae were measured using ImmunoCAP (ThermoFischer, Uppsala, Sweden), and the percent inhibition was calculated. ImmunoCAP inhibition assay was repeated using different batches of SLIT and SCIT reagents.
The protein concentration in the Wolwopharma® HDM SLIT reagent (447.70 µg/mL) was higher than that in Staloral® (292.58 µg/mL) or SLITone® (1.71 µg/mL) (Fig. 1A). However, the concentrations of group 1 and group 2 major allergens of HDM were higher in Staloral® reagent than in SLITone® or Wolwopharma® (Fig. 1B). The concentration of group 1 major allergens in Staloral® HDM SLIT reagent (132.03 µg/mL) was 33- to 44.5- fold higher than that in SLITone® (4.00 µg/mL) or Wolwopharma® (2.97 µg/mL). Staloral® HDM SLIT reagent also had a higher concentration of group 2 major allergen than that of SLITone® or Wolwopharma®. The concentration of group 2 major allergen in Staloral® SLIT reagent (14.67 µg/mL) was 4.2-fold and 5.1-fold higher than in SLITone® (3.46 µg/mL) and Wolwopharma® (2.91 µg/mL), respectively.
The ELISA inhibition study against HDM-specific IgE showed that the allergenicity of Staloral® SLIT reagent is 8.5-fold and 21-fold higher than that of SLITone® and Wolwopharma®, respectively. We also compared the allergenic potency of SLIT reagents with 10,000 AU Hollister-Stier SCIT HDM reagents. The allergenic potency of Staloral (300 IR/mL) is equal to Hollister-Stier D. farinae SCIT (10,000 AU/mL) and 78% of Hollister-Stier D. pteronyssinus SCIT reagent (10,000 AU/mL) (Fig. 2A, B). In addition, we performed an ImmunoCAP inhibition test (Fig. 3), which showed results similar to the ELISA inhibition assays.
The recommended volume of Staloral® is 400 µL, compared to 200 µL of SLITone® and Wolwopharma®. Thus, based on the ELISA inhibition test, the maintenance dosage of Staloral® HDM reagent is 17-fold higher than that of SLITone® and 42-fold higher than that of Wolwopharma®. Based on group 1 major allergen levels determined by two-site ELISA, the Staloral® HDM SLIT maintenance dose is 66.0-fold higher than SLITone® and 89.5-fold higher than Wolwopharma®. Group 2 major allergen levels in Staloral® reagent are 8.5-fold higher than in SLITone® and 10.1-fold higher than in Wolwopharma®.
This study showed that, based on IgE binding affinity, a more than 42-fold difference can exist between the maintenance dosages of commercially available SLIT reagents. This finding may be due to the lack of consensus regarding the optimal maintenance dose of HDM SLIT. Our study emphasizes the practical importance of coming to a consensus on the appropriate maintenance dosage of HDM SLIT reagents. For HDM respiratory allergy patients, guidelines recommend administration of 2,000 AU for the SCIT maintenance dose.7 As the allergen potencies of 300 IR/mL and 10,000 AU/mL are similar in this study, the daily maintenance dose of Staloral® is twice as high as the monthly maintenance dose of SCIT, suggesting that the monthly dosage of Staloral® is 60-fold greater than that of SCIT. We showed the cumulative doses of total protein and major allergens after 28 days of treatment using 4 different products (Table 2).
Our results are consistent with those of other researchers. Mösges et al.12 compared the allergen potencies of Staloral® and SLITone® HDM SLIT reagents using the skin prick test and found that 1,000 STU of SLITone® is equivalent to 78 IR/mL of Staloral®, suggesting that the maintenance dose of Staloral® is 22 times higher than that of SLITone®. Larenas-Linnemann et al.13 also reported marked differences in the potencies of 4 European commercial HDM SLIT reagents, and showed that high-dose SLIT is not commonly used in Europe.
A randomized clinical study using HDM allergic asthma patients showed that high-dose SLIT (daily maintenance dose containing 70 µg of Der f 1) could increase the bronchial threshold to allergen challenge and D. farinae-specific IgG4, but low-dose SLIT (daily maintenance dose containing 1 µg of Der f 1) could not. The researchers suggested that the efficacy of SLIT in treating HDM allergy may also be dose-dependent, and high-dose SLIT may be effective.16 For grass pollen allergic patients, the dose-response relation has been well-proven by randomized double-blind clinical trials using 5-grass pollen SLIT tablets.9,10 However, a recent systematic review study failed to find a significant difference in the efficacy of SLIT using different maintenance dose. This is partly due to the difficulty in determining the dose of major allergens to administer,1 and partly because the optimal maintenance dose of HDM SLIT treatment is not yet known.16 The review study may suggest that the therapeutic equivalence dose can vary widely. Slater et al.,17 showed that therapeutic equivalence is achieved over a 10-fold range of allergen concentrations based on SCIT studies using dose-response data.
Previous studies reported that when the allergen concentration of SCIT is increased fourfold, adverse reactions increase about 5% to 10%.18 However, although local adverse reactions were more common using the lower dose, systemic adverse reactions to SLIT were not dose dependent.3 Adding immunologic adjuvants to the SLIT agents may increase the clinical efficacy and decrease the adverse reactions. Also, clinical efficacy can be sustained if the allergen capacity is low.19
The allergen potency of an immunotherapeutic extract can be evaluated by 2 methods. The first is based on calculating the concentration of major allergens using two-site ELISA or the radial immunodiffusion method. Another method involves comparing total allergen potency, usually by an ELISA inhibition test or an in vivo skin test.20,21 In this study, we found an up to 42.5-fold difference in total allergen potency but a smaller difference (up to 17.4-fold) in the concentration of group 1 major allergens of HDM between reagents. This result is consistent with other reports that have shown marked discrepancies in the major allergen concentrations in extracts even when they had equal allergen potency.13,20 For measurements of group 1 and 2 major allergens, we used a two-site ELISA kit from Indoor Biotechnologies using monoclonal antibodies. Monoclonal antibodies are usually specific and cannot detect all isoallergens of a major allergen,22 and this method has not yet been validated for standardization of allergen extracts.23 Thus, the measurement may be different when using another two-site ELISA kit, and this might represent a limitation of our study. This issue was unambiguously recognized by the CREATE (Certified References for Allergens and Test Evaluation) consortium.23,24 They have since tried to develop acceptable recombinant major allergens, as well as two-site ELISA kits for each major allergen, to be used as international standard materials. In this context, the European Pharmacopoeia Commission has recently adopted only 2 recombinant allergens as Chemical Reference Substances. They are currently used as reference standards for the determination of Bet v 1 and Phl p 5 concentrations by ELISA. However, guidelines for SCIT recommend 5-20 µg for the maintenance dose, emphasizing the importance of the concentration of major allergens.25 We are not sure yet whether major allergen concentration or total allergen potency is more important in the efficacy of immunotherapy. Our study also found that allergen potencies of SLIT reagents are not correlated with protein content. The protein concentration of Staloral® was 292.58 µg/mL, and the concentration of group 1 major allergens was responsible for 45.1% (132.08 µg/mL) of whole protein concentration, indicating the high quality of raw material used for manufacturing this SLIT reagent.
Our results reveal marked differences in the allergen potencies of different SLIT reagents. However, as we did not study their clinical efficacy, our results should not be taken as an indication of their clinical efficacy. Our data strongly suggest that further dose response studies to determine the clinical efficacies and maintenance dosages of HDM SLIT reagents studies are urgent. Our results also showed that high-dose SLIT is not yet in common use for HDM respiratory allergy patients who have been treated with SLIT.
Figures and Tables
Fig. 1
Protein concentrations (A) and major allergen concentrations (B) in the house dust mite sublingual immunotherapy reagents and Hollister-Stier subcutaneous immunotherapy reagents. HS, Hollister-Stier; Wolwo, Wolwopharma.
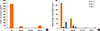
Fig. 2
ELISA inhibition of D. pteronyssinus-specific IgE (A) and D. farinae-specific IgE (B) with house dust mite sublingual immunotherapy reagents and Hollister-Stier subcutaneous immunotherapy reagents. HS, Hollister-Stier; Wolwo, Wolwopharma (A: D. farinae 10,000 AU/mL, B: D. pteronyssinus 10,000 AU/mL).

Fig. 3
ImmunoCAP inhibition of D. farinae-specific IgE with house dust mite sublingual immunotherapy reagents and Hollister-Stier subcutaneous immunotherapy reagents. HS, Hollister-Stier; Wolwo, Wolwopharma (D. farinae 15,000 AU/mL and D. pteronyssinus 15,000 AU/mL mixture).

Table 1
Allergen potencies, recommended administration volumes, and maintenance dosages of three commercially available SLIT reagents according to their manufacturers' instruction sheets

Table 2
Cumulative doses of total protein and major allergens after 28-day treatment using 4 different products
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Healthcare Technology R&D project, Ministry of Health and Welfare, Republic of Korea (A092076).
References
1. Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011; 66:740–752.
2. Compalati E, Passalacqua G, Bonini M, Canonica GW. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta-analysis. Allergy. 2009; 64:1570–1579.
3. Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009; 64:Suppl 91. 1–59.
4. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI). A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013; 5:277–282.
5. Calderón MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012; 67:302–311.
6. Norman PS. An overview of immunotherapy: implications for the future. J Allergy Clin Immunol. 1980; 65:87–96.
7. Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007; 120:S25–S85.
8. Ewbank PA, Murray J, Sanders K, Curran-Everett D, Dreskin S, Nelson HS. A double-blind, placebo-controlled immunotherapy dose-response study with standardized cat extract. J Allergy Clin Immunol. 2003; 111:155–161.
9. Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 117:802–809.
10. Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007; 120:1338–1345.
11. Bousquet J, Bieber T, Fokkens W, Humbert M, Kowalski ML, Niggemann B, et al. Consensus statements, evidence-based medicine and guidelines in allergic diseases. Allergy. 2008; 63:1–4.
12. Mösges R, Pasch N, Schlierenkämper U, Lehmacher W. Comparison of the biological activity of the most common sublingual allergen solutions made by two European manufacturers. Int Arch Allergy Immunol. 2006; 139:325–329.
13. Larenas-Linnemann D, Esch R, Plunkett G, Brown S, Maddox D, Barnes C, et al. Maintenance dosing for sublingual immunotherapy by prominent European allergen manufacturers expressed in bioequivalent allergy units. Ann Allergy Asthma Immunol. 2011; 107:448–458.
14. Cox L, Jacobsen L. Comparison of allergen immunotherapy practice patterns in the United States and Europe. Ann Allergy Asthma Immunol. 2009; 103:451–459.
15. Larenas-Linnemann D, Cox LS. Immunotherapy and Allergy Diagnostics Committee of the American Academy of Allergy, Asthma and Immunology. European allergen extract units and potency: review of available information. Ann Allergy Asthma Immunol. 2008; 100:137–145.
16. Bush RK, Swenson C, Fahlberg B, Evans MD, Esch R, Morris M, et al. House dust mite sublingual immunotherapy: results of a US trial. J Allergy Clin Immunol. 2011; 127:974–981.e1-7.
17. Slater JE, Pastor RW. The determination of equivalent doses of standardized allergen vaccines. J Allergy Clin Immunol. 2000; 105:468–474.
18. Cox L, Esch RE, Corbett M, Hankin C, Nelson M, Plunkett G. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol. 2011; 107:289–299.
19. Francis JN, Durham SR. Adjuvants for allergen immunotherapy: experimental results and clinical perspectives. Curr Opin Allergy Clin Immunol. 2004; 4:543–548.
20. van Ree R. Indoor allergens: relevance of major allergen measurements and standardization. J Allergy Clin Immunol. 2007; 119:270–277.
21. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011; 52:393–400.
22. Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, et al. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002; 32:1042–1047.
23. van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008; 63:310–326.
24. Chapman MD, Ferreira F, Villalba M, Cromwell O, Bryan D, Becker WM, et al. The European Union CREATE project: a model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008; 122:882–889.e2.
25. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102:558–562.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download