Abstract
Cephalosporins can cause a range of hypersensitivity reactions, including IgE-mediated, immediate reactions. Cephalosporin allergy has been reported with use of a specific cephalosporin, as a cross-reaction between different cephalosporins or as a cross-reaction to other β-lactam antibiotics. Unlike penicillins, the exact allergenic determinants of cephalosporins are less well understood and thus, standardized diagnostic skin testing is not available. Nevertheless, skin testing with diluted solutions of cephalosporins can be valuable in confirming IgE-mediated hypersensitivity reactions. In vitro tests are in development using recent technological advances and can be used as complementary tests. However, they are not commonly used because of their reduced sensitivity and limited availability. In selected cases of inconclusive results in both skin tests and IgE assays, a graded challenge or induction of drug tolerance with the implicated cephalosporin should be performed.
Cephalosporins can cause a range of hypersensitivity reactions from mild, cutaneous reactions to life-threatening anaphylaxis in patients with IgE-mediated allergy.1,2,3,4,5 As the number of cephalosporin prescriptions has increased, the prevalence of immediate hypersensitivity reactions to cephalosporins also appears to be on the rise.6 Allergic reactions to cephalosporins have been considered primarily in conjunction with penicillin allergy. However, cephalosporin allergy can occur to a specific cephalosporin, a group of cephalosporins, or as a cross-reaction to other β-lactam antibiotics.3,6,7,8,9,10,11,12
The estimated prevalence of hypersensitivity to cephalosporins is 1%-3% in the general population.13,14 However, a greater number of patients claim to be allergic to cephalosporins, and they are not treated with these medications due to the fear that they may be at risk of anaphylaxis. In a study by Lee et al.,15 patients labeled as allergic to penicillin and/or cephalosporin were more likely to be treated with vancomycin or levofloxacin compared to patients without any antimicrobial allergy. Use of these alternative agents may contribute to the development and spread of multiple drug-resistant bacteria and leads to higher health care costs.
Unlike penicillins, degradation of cephalosporins creates multiple, unstable antigenic fragments that have yet to be fully identified.8 In the absence of validated reagents for cephalosporin skin testing, physicians have used dilutions of native cephalosporins to detect the presence of specific IgE antibodies, which may result in a false-negative skin test. In addition, since cephalosporin skin tests are not standardized, there is the potential for false positives. Consequently, cephalosporin skin tests have a limited clinical value, often creating a diagnostic dilemma.
In this article, we discuss the immunogenicity and cross-reactivity of cephalosporin allergy and review the diagnosis and management of IgE-mediated hypersensitivity reactions to cephalosporins.
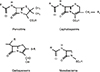
Cephalosporins, except for their 7-methoxy analogs cephamycins, are semisynthetic derivatives of cephalosporin C, which was initially obtained from a culture of the fungus Cephalosporium acremonium.7,8 All β-lactam antibiotics have a core, four-membered cyclic amide β-lactam ring structure (Fig. 1). In penicillins, the β-lactam ring is attached to a thiazolidine ring, which together comprises the 6-amino-penicillanic acid nucleus. In cephalosporins, the β-lactam ring is attached to a dihydrothiazine ring, making a 7-amino-cephalosporanic acid nucleus. Both classes of drugs have an R1 side chain attached to the β-lactam ring and cephalosporins have an additional R2 side chain on the dihydrothiazine ring. The nature of the R group determines the drug's stability to enzymatic or acidic hydrolysis, and affects its antibacterial spectrum. Cephalosporins are grouped into five generations, largely on the basis of bacterial susceptibility patterns and resistance to β-lactamases.7,8,16
Carbapenems are synthetic β-lactam antibiotics that differ from the penicillins in that the sulfur atom of the thiazolidine ring has been externalized and replaced by a carbon atom. As a result of this structure, carbapenems have the potential for cross-reactivity with penicillins,17 but can resist hydrolysis by most β-lactamases. Monobactams are unique because the β-lactam ring is not attached to another ring structure.
Drugs that are most likely to cause an allergic reaction are those that combine covalently with proteins.18 However, most antibiotics in their native state are not chemically reactive. To act as haptens, antibiotics must first be converted to reactive intermediates by an enzymatic metabolic reaction known as hepatic biotransformation phase I.19 Hypersensitivity reactions occur when reactive drug intermediates bind to host proteins before phase II enzymes can bioinactivate the intermediate to non-reactive products.19 Important exceptions to this mechanism are β-lactam antibiotics, which can cause hypersensitivity reactions without being metabolized enzymatically.
In penicillins, the condensed fusion of the β-lactam ring structure with a thiazolidine ring causes increased tension within the β-lactam ring, resulting in spontaneous opening of the ring under physiologic conditions. This allows the highly reactive carbonyl group to bind easily to the amino groups of lysine residues present on adjacent proteins, forming the highly stable conjugate protein, benzyl penicilloyl.20,21,22 Benzyl penicilloyl comprises approximately 95% of cell-bound penicillin and is the major antigenic determinant.23 Penicillin molecules that do not form penicilloyl linkages degrade to a variety of derivatives that are also capable of binding to host proteins.24 Of these degradation products, three components--penicilloate, penilloate, and native penicillin itself--comprise all relevant allergenic determinants not covered by penicilloyl and collectively are called the minor antigenic determinants. Use of benzyl penicilloyl (Pre-Pen) alone identified ~80% of patients allergic to penicillin.25 Native penicillin G is the most important commercially available minor determinant. In a large cooperative study of 3,000 patients sponsored by the American Academy of Allergy, only 3% of patients with a past history of allergy and negative skin tests to benzyl penicilloyl and penicillin G had a reaction when they were challenged with penicillin or derivatives.26
Similarly, opening of the β-lactam ring of cephalosporins produces a protein conjugate, the cephalosporyl determinant. However, unlike penicillins, which preserve the thiazolidine ring upon degradation, the degradation process of cephalosporins involves destruction of the dihydrothiazine ring, as well as the β-lactam ring. Therefore, the cephalosporyl determinant is highly unstable and degrades further into multiple antigenic fragments as the dihydrothiazine ring ruptures.8 This makes isolation and characterization of the different antigenic elements of cephalosporin difficult.
Although the exact antigenic determinants of cephalosporins are not well understood, there is evidence that cephalosporins generate unique structures capable of provoking IgE-mediated immunologic responses. Various in vitro studies have demonstrated that IgE antibodies reacting with cephalosporins can detect a wide range of specificities, ranging from a small moiety, such as a portion of a side chain, to a full side chain, a combination of a side chain and part of the β-lactam ring, or even the whole cephalosporin molecule.9,12,27,28,29 The different singularities in the recognition of a cephalosporin molecule have also been demonstrated in clinical studies.3,6,10,11
Retrospective studies prior to the 1980s reported that patients who had a history of penicillin allergy reacted to administration of a cephalosporin ~10% of the time.30,31,32,33 These reports tended to involve first- or second-generation cephalosporins and rely on history, not skin testing. The high rate of cross-reactivity in these studies was in contrast to a more recently reported risk of cephalosporin allergy of <1% in patients who had a past history of an immediate reaction to penicillins but did not undergo penicillin skin testing,23 and ~2% in patients who had a past history and positive skin test result.13,34,35,36 Several facts should be considered in the interpretation of these earlier studies. The high rate of cross-reactivity in these studies is likely due to the fact that some of the first-generation cephalosporins were contaminated with trace amounts of penicillin.37 In addition, because drug-allergic patients may have an increased risk of developing allergic reactions to unrelated, non-cross-reacting compounds,38 such reactions to cephalosporins in penicillin-allergic individuals may not reflect true cross-reactivity between the 2 classes of antibiotics. Furthermore, the major cephalosporins prescribed prior to the 1980s were cephalothin and cephaloridine, which have side chains similar to benzyl penicillin.7 Cross-reactivity between first-generation cephalosporins and penicillins was demonstrated in a recent meta-analysis.39 To summarize, a significant increase in allergic reactions to cephalothin (odds ratio [OR]=2.5; 95% confidence interval [CI]=1.1-5.5), cephaloridine (OR=8.7; 95% CI=5.9-12.8), cephalexin (OR=5.8; 95% CI=3.6-9.2), and all first-generation cephalosporins plus cefamandole (OR=4.8; 95% CI=3.7-6.2) were observed in penicillin allergic patients. However, cross-reactivity was negligible between penicillins and second- and third-generation cephalosporins.
Because cephalosporins and penicillins share a common β-lactam ring, one might expect that this structure is responsible for their cross-reactivity. However, most patients with immediate reactions to cephalosporins, and no history of reacting to penicillins, will tolerate penicillins, suggesting that side chains, rather than the β-lactam ring, play an important role in immunologic cross-reactivity. Consistent with this idea, there is experimental and clinical evidence that the R1 side chain shared by some penicillins and cephalosporins is the determining factor in their cross-reactivity.40,41 The aminopenicillins share the same R1 side chain as several first- and second-generation cephalosporins. For example, amoxicillin has the same side chain as cefadroxil, cefprozil, and cefatrizine; ampicillin has the same side chain as cefaclor, cephalexin, cephradine, cephaloglycin, and loracarbef. Miranda et al.42 evaluated cross-reactivity between amoxicillin and cephlosporins, specifically cefadroxil, which contains an identical side chain to that of amoxicillin, and cefamandole, which contains a different side chain to that of amoxicillin. Among 21 patients who had documented amoxicillin allergy but negative results to penicillin skin testing, 8 patients (38%) had a positive response to cefadroxil and none reacted to cefamandole. In addition, in vitro radioallergicsorbent (RAST) inhibition studies indicated the epitope implicated in this type of sensitization might be located on the side chain of the amoxicillin molecule. In a similar study, Sastre et al.43 reported a lower rate of cross-reactivity with only 2 of 16 patients (12%) allergic to amoxicillin but tolerant to penicillin G exhibiting an immediate allergic reaction when challenged with cefadroxil, which shares an identical side-chain. In the case of ampicillin, Audicana et al.40 reported that 31% of patients with ampicillin allergy reacted to cephalexin, which have identical R1 side chains. Taken together, a significant portion of patients, who proved to be selectively allergic to aminopenicillins and able to tolerate penicillins, reacted to cephalosporins which have an identical side chain to aminopenicillins. Therefore, patients with amoxicillin or ampicillin allergy should avoid cephalosporins with an identical side chain or receive them through induction of drug tolerance procedures.44
Cross-reactivity has been reported to occur infrequently between penicillins and cephalosporins with different side chains. Blanca et al.41 reported that 17 of 19 patients (89.4%) allergic to penicillins tolerated challenges to a therapeutic dose of cephaloridine and cefamandole, which have different side chains to penicillin. Moreover, Novalvos et al.45 reported that all of 41 patients allergic to penicillins showed negative results to cephalosporin skin testing and tolerated challenges to a therapeutic dose of cephalosporins that contain a different side chain to the culprit penicillin. Their study indicates that, in penicillin-allergic subjects, the risk of an allergic reaction to cephalosporins seems to be very low, provided that cephalosporins with a different side chain to the culprit penicillin are used.
A small moiety shared by cephalosporins and penicillins may be antigenically important. A population of IgE antibodies recognizing a methylene group present in both cephalothin and benzylpenicillin was identified in patient sera.29 Positive skin tests to both benzylpenicillin and cephalothin were observed in such patients. Finally, a small percentage of patients with primary cephalosporin reactions may be sensitized to the β-lactam core that is common to both cephalosporins and penicillins.46
Side chains may be the most common source of cross-reactivity among cephalosporins (Tables 1, 2). During cephalosporin degradation, the rupture of the dihydrothiazine ring leads to expulsion of the R2 group. Thus, the intact R1 group appears to be more important for the cross-reactivity of cephalosporins, compared to the lost R2 group.5,9,22 Multiple case reports support these conclusions.11,47,48 However, there is in vitro and clinical evidence that R2 side chains also contribute to the cross-reactivity among cephalosporins.9,28,49
A small moiety also may play a role in cross-reactivity among cephalosporins. Some cephalosporins share the same moiety at the R1 side chain position. As examples, ceftriaxone, cefuroxime, cefotaxime, and cefepime each have a methoxyimino group within the R1 side chain and cross-reactivity was observed in patients allergic to these agents.5 In contrast, ceftazidime has an R1 side chain that is slightly different from ceftriaxone, cefotaxime, cefepime, and cefuroxime. The R1 side chain of ceftazidime has an alkoxyimino group that has greater steric hindrance than the methoxyimino moiety, and so would not be expected to be recognized by the same IgE molecules.50 Consistent with this idea, a previous study found a significant degree of cross-reactivity between cefotaxime and ceftriaxone (OR=7.0, 95% CI=2-24), but not between these cephalosporins and ceftazidime.5 However, it should be noted that some subjects experienced immediate hypersensitivity reactions to ceftazidime, as well as to cefotaxime and/or ceftriaxone.46,51
Although aztreonam, a clinically available monobactam, contains a monocyclic β-lactam core, it has minimal to no cross-reactivity with penicillins.16,52,53 However, aztreonam shares a side chain structure with ceftazidime, and clinical cross-reactivity has been reported between the 2 drugs.52,54,55 Nevertheless, not all ceftazidime-allergic patients react to aztreonam,56,57 and vice versa.58
Unlike aztreonam, carbapenems have a high incidence of cross-reactivity with penicillins. Of 20 subjects with positive skin tests to one or more penicillin determinants, 50% reacted to imipenem reagents.17 However, clinical cross-reactivity between carbapenems and cephalosporins appears to be rare. In a study of 98 patients with a history of immediate reactions and positive skin test results to cephalosporins, only one patient was skin test-positive to carbapenems (meropenem and imipenem/cilastatin).56 Of the 97 patients with negative skin tests to carbapenems, 96 tolerated graded challenges to carbapenems. The one remaining patient developed a mild urticaria 30 minutes after the full dose of carbapenems.
Cephalosporins cannot simply be considered a group of compounds with a common allergenic determinant structure. Sometimes, patients suffer an immediate hypersensitivity reaction to a certain cephalosporin only, with tolerance to other β-lactam antibiotics. There are cases of selective immediate hypersensitivity to cephalosporins, such as cefazolin, cefuroxime, ceftriaxone, cefixime, cefodizime, or cefepime, in which selective responses have been demonstrated by skin testing, IgE assay, and challenges with other β-lactams.10,51,59,60,61,62 The proposed mechanism is recognition by IgE antibodies of the entire cephalosporin molecule.
Past history of penicillin or cephalosporin allergy is the most important risk factor for reacting to cephalosporins. Penicillin-allergic patients have a threefold increased risk of adverse reaction to any medication.63 The risk of cephalosporin allergy has been reported to be ~2% in patients with a past history of immediate reactions and positive skin tests to penicillins.13,34,35,36 However, it should be emphasized that the majority of the reactions were cases of anaphylaxis, some of which were fatal.64 Patients with a history of cephalosporin allergy are at increased risk of subsequent reaction to the same or related cephalosporins.4,6,46,51 In a previous study of 30 patients with immediate reactions to one or more of the third-generation cephalosporins, 26 patients (86.7%) had a positive skin test for cephalosporins with negative skin and RAST tests to penicillin determinants. Two patterns of cephalosporin allergy were observed: one characterized by a response only to the culprit cephalosporin (57.7%) and the other by positive responses to different cephalosporins, including the responsible cephalosporins (42.3%).51 In a similar study, Antunez et al.6 reported that 36.8% of patients with cephalosporin allergy reacted to another cephalosporin.
A thorough history is an essential component of the evaluation of patients with suspected cephalosporin allergy. The history helps guide the clinician in the choice of diagnostic tests and the best therapeutic strategy. The most important aspects of a patient history are as follows: 1) name of the cephalosporin prescribed and exact structure of R-group side chains; 2) dose and route of the medication; 3) presenting symptoms and signs, and extent of organ system involvement; 4) timing of the reaction in relation to administration of the medication; 5) concurrent medications at the time of reactions; 6) past history of allergic reactions to other β-lactam antibiotics; 7) type of illness for which the antibiotic was prescribed; 8) any prior or subsequent history of exposure to the same drug or structurally similar drugs. However, even though history is helpful, it cannot reliably identify allergies to cephalosporins. This can be best accomplished by skin testing and, occasionally, a graded challenge.
A positive skin test result to a cephalosporin suggests that drug-specific IgE antibodies may be present. However, since cephalosporin skin tests are not standardized, there is the potential for false-positive tests from non-specific irritant reactions. To avoid this possibility, the highest concentration of a drug that would not elicit an irritant skin reaction in subjects without drug allergy should be established for each drug. Empedrad et al.65 reported a concentration of 10 mg/mL ceftriaxone, cefotaxime, cefuroxime, and ceftazidime and a concentration of 33 mg/mL cefazolin was non-irritating to the skin of subjects with no history of drug allergy. In a recent position paper, the ENDA/EAACI Drug Allergy Interest Group recommended a concentration of 2 mg/mL for cephalosporin skin tests.66 However, several studies indicate that for cefuroxime, ceftriaxone, cefotaxime, ceftazidime, cefazolin, cephalexin, cefaclor and cefatrizine, concentrations up to 20 mg/mL are non-irritant and might improve sensitivity without affecting specificity.67,68
Cephalosporin skin testing is usually performed by skin prick testing followed by intradermal testing using the native drug, diluted in normal saline, at non-irritating concentrations. For patients with a history of severe anaphylaxis to a cephalosporin, solutions for initial skin testing can be diluted 10- to 1,000-fold further to reduce the risk of inducing a systemic reaction. A positive skin test to a cephalosporin may be defined as a wheal >3 mm in diameter for prick and >5 mm for intradermal testing, with surrounding erythema that develops in 10-15 minutes.46,69 Romano et al.47 found that a concentration of 2 mg/mL injectable cephalosporins, mixed in normal saline, was non-irritating to the skin. In a subsequent study, they confirmed cephalosporin allergy in 29 of 30 patients with a history of immediate hypersensitivity to cephalosporins, suggesting that skin testing at such a concentration is a sensitive tool for cephalosporin allergy.51
Although skin testing with diluted solutions of cephalosporins can be valuable in confirming IgE-mediated hypersensitivity to cephalosporins, its limitations must be considered. The sensitivity of cephalosporin skin testing has been estimated in three European studies of children and/or adults.6,46,70 In those studies, skin testing was positive in 72% (31/43), 31% (39/127), and 70% (53/76) of patients with immediate reactions to cephalosporins, respectively. Further studies are needed to fully establish the sensitivity of cephalosporin skin testing. In addition, the positive predictive value of skin testing has not been precisely defined, because this would require administering the drug to large numbers of skin test-positive patients to confirm their reactivity. Furthermore, the negative predictive value of cephalosporin skin testing has not been established. If prick and intradermal test responses are negative, it suggests that the patient does not have specific IgE antibodies to the cephalosporin in its native state, although the patient could still have IgE antibodies against a degradation product-protein complex. Therefore, a negative result should not be interpreted as proof that allergy is not present. As an example, in a study of adults with immediate reactions to cephalosporins, 8 of 13 patients with negative skin tests agreed to undergo challenges with the suspect drug, and 2 patients (25%) reacted.46 This is in contrast to the negative predictive value of penicillin skin tests, which has been reported to be 97%-99%.71,72
Recently, Yoon et al.73 investigated the validity of skin tests for predicting immediate hypersensitivity to cephalosporins in subjects with no history of allergy to β-lactam antibiotics. Intradermal skin tests were performed with four cephalosporins, one selected from each generation of cephalosporins, as well as penicillin G, in 1,421 patients who required a preoperative antibiotic. Irrespective of the skin test results, each patient received an intravenous challenge dose of one of the tested cephalosporins with careful observation. Seventy-four patients (5.2%) were skin test-positive to at least one cephalosporin, and none of them displayed immediate hypersensitivity to the challenge, while four of the patients who had negative skin tests developed an immediate hypersensitivity reaction. These results indicate that, in patients with no history of β-lactam allergy, skin testing with cephalosporins before its administration is not useful for predicting immediate hypersensitivity.
When evaluating patients with immediate cephalosporin reactions, the following reagents should be included in the skin-testing panel: the cephalosporin that caused the reaction, one or more alternative cephalosporins with different R groups from the culprit drug, and the penicillin reagents, ampicillin (20 mg/mL) and amoxicillin (20 mg/mL). The classic penicillin reagents include penicilloyl-polylysine (Pre-Pen, 5×10-5 mM) and the minor determinant mixtures (MDM, 2×10-2 mM).66 Native penicillin G is the only minor determinant that is commercially available and can be used for skin testing at a concentration of 10,000 U/mL.
Two techniques, radioimmunoassay (RIA) and fluoroenzymeimmunoassay (FEIA), have been used in research studies to detect specific IgE antibodies to a limited number of β-lactam antibiotics.74 Sensitivity and specificity of assays for the penicillin-specific IgE were reported to be 29%-68% and 97%-100%, respectively.27,28,29,41,46,51,75 However, studies of the other β-lactams, including cephalosporins are limited. The sensitivity of the cephalosporin-specific IgE assay has been reported to be 30% and 74.3%, respectively, in 2 clinical studies of patients who had a history of immediate reactions to cephalosporins.46,51 Fontaine et al.75 investigated the diagnostic value of specific IgE determination in the diagnosis of immediate β-lactam allergy.75 The specificity of FEIA ranged from 83.3%-100% and sensitivity from 0%-25% depending on the initial clinical manifestations. The specificity of RAST was between 66.7%-83.3% and sensitivity between 42.9%-75%. In the subgroup of patients with anaphylactic shock and negative skin tests, the sensitivity and specificity of RAST were 75% and positive and negative predictive values were 45.5% and 77.1% with FEIA and 38.5% and 81.5% with RAST, respectively.75 Their study suggested that, owing to high specificity, serum-specific IgE assays may be useful to avoid a drug provocation test in the patients with a history of β-lactam anaphylaxis and negative skin tests.
In the case of penicillins, IgE titer can be decreased below the cut-off value over time and sensitivity can be reduced more rapidly than skin tests.76 However, the phenomenon is not as well documented in cephalosporin allergy as it is in penicillin allergy, and the rate of loss of sensitization is not known.77,78 Additionally, a test result could be falsely positive if patients have high total IgE titers and it could be falsely negative if patients have high IgG antibody levels.79 Furthermore, the amount of specific IgE may not reflect the severity of clinical reactions.79
At present, an IgE immunoassay is commercially available to benzyl penicillin, phenoxymethyl penicillin, ampicillin, amoxicillin, and cefaclor (ImmunoCAP, ThermoFisher, Uppsala, Sweden).5
The basophil activation test (BAT) is a quantitative measurement of the cell surface protein, CD63, on activated basophils by flow cytometry after stimulation with culprit drugs in vitro.80,81 CD203c is another basophil activation marker recently shown to be a more sensitive marker of basophil activation than CD63 for the diagnosis of amoxicillin allergy.82
BAT has proven to be a sensitive and specific for the diagnosis of several IgE-mediated allergies.83,84 However, basophil activation by drugs is less remarkable than protein allergens. The sensitivity and specificity of BAT in patients with β-lactam allergy have been reported to be 50%-60% and >90%, respectively.81,85,86 However, data concerning its sensitivity and specificity are lacking. Further studies are needed before it can be employed as a diagnostic tool.
Penicillin is a class of antibiotics with a relatively high frequency of allergy, reported to be 10%. However, 90% of patients with a history of penicillin allergy have negative results in penicillin skin testing.71,72 This observation is due in part to the fact that penicillin-specific IgE antibody levels decrease over time.87 In addition, many patients were probably mislabeled as being allergic at the time of their reaction because clinical features of an underlying illness can be confused for a penicillin-induced reaction.
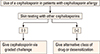
Penicillin skin testing can help guide physicians in their approach to patients with a history of penicillin allergy that requires treatment with cephalosporins (Fig. 2). Patients who have negative results in penicillin skin testing are at no greater risk of an allergic reaction and can safely receive cephalosporins.4 This group may include the vast majority of individuals labeled penicillin allergic.71,72 On the other hand, administration of cephalosporins to patients who have positive results in penicillin skin testing should be approached more cautiously. If possible, such patients should be skin-tested with a non-irritating concentration of the cephalosporin before treatment.34 Patients who have positive results in cephalosporin skin testing should be either not treated with the cephalosporin or desensitized if alternative drugs are not available.7 Because the negative predictive value of cephalosporin skin testing is not known, the cephalosporin should be administrated by a graded challenge in patients who showed positive results to penicillin and negative results to cephalosporin in skin testing.4,7 When patients with a history of penicillin allergy are given a cephalosporin directly without penicillin skin testing, the chance of reacting to the cephalosporin is <1%.7,23 However, since cases of fatal anaphylaxis have been reported following cephalosporin administration, a graded challenge should be strongly considered.64,88,89
Most patients with immediate reactions to cephalosporins and no history of reacting to penicillins, will tolerate penicillins. However, some patients react to both groups of drugs. Therefore, skin testing with the penicillin reagent is indicated in such patients to guide management (Fig. 3).90 Negative results in penicillin skin testing indicate that the patient's reaction to the cephalosporin was probably due to a unique cephalosporin determinant. Therefore, the patient is not at increased risk for reacting to a penicillin, provided that the penicillin does not share a side chain with the culprit cephalosporin.6 Positive results indicate that the patient may be reactive to the β-lactam core structure or side chains that are shared by the penicillin and the culprit cephalosporin. The patient may be treated with a non-β-lactam antibiotic or desensitized to the desired penicillin. If penicillin skin testing is not available, it is advisable to select a penicillin that does not have a similar side chain to that of the culprit cephalosporin, and to perform skin testing with that penicillin in its native form. If the results are negative, the patient may be treated with a graded challenge to the penicillin.
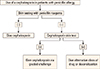
In patients allergic to cephalosporins, skin testing should be performed with one or more desired cephalosporins that have a different side chain, especially in the R1 position, from the culprit one (Fig. 4). If the results are positive, the patient should be assumed to be allergic to the desired cephalosporin and it should only be administered using a desensitization protocol. If the results are negative, then the desired cephalosporin can be administered by a graded challenge.3 Rarely, a patient may require the same cephalosporin to which there is evidence of IgE-mediated allergy. A formal desensitization protocol should be performed in this situation.
When there is a definite medical indication for the cephalosporin in question, either a graded challenge or desensitization procedures may be considered. The choice of procedures depends on the likelihood of allergy at the time. A graded challenge is more appropriate for a patient who is unlikely to be allergic to the implicated drug, whereas desensitization should be considered in a patient who is likely to be allergic to the implicated drug.
A graded challenge is perhaps the most reliable in vivo test to prove or disprove unequivocally whether an antibiotic allergy exists. The challenge procedure does not modify a patient's response to a drug, so patients who tolerate it prove that they are not allergic. The most common reason for performing a graded challenge is to confirm that a patient can safely receive an alternative cephalosporin, other than the culprit drug, to which the patient is skin-test negative. A graded challenge may also be useful in the penicillin-allergic patients requiring a cephalosporin who showed a negative result in cephalosporin skin testing.
In the case of immediate reactions to β-lactam antibiotics, negative skin test results should be interpreted in light of the time elapsed since the subject's last exposure to the drug. Skin testing with cephalosporins is more likely to be positive the less time has elapsed since the IgE-mediated hypersensitivity reaction.36,46,70 Therefore, it is advisable to perform challenges with the suspect cephalosporin in subjects with a past history of cephalosporin allergy who have negative skin test results when evaluated more than 6 months after their last reaction. It should be noted that resensitization has been observed in a few patients retested about 1 month after either a negative or positive challenge.36,46,70 As a result, it is recommended to retest such patients after 1 month to exclude a possible resensitization after loss of sensitivity.46,91,92
Provocation testing with the implicated cephalosporin can be conducted according to the recommendation of both the American and European guidelines.23,93 A graded challenge typically involves 2 or 3 steps. The starting dose for a graded challenge is usually 1/100 of the full dose, and 10-fold increasing doses are administered every 30 to 60 minutes until the full therapeutic dose is reached.23 A lower starting dose should be used in patients with a history of severe reactions. A graded challenge can be dangerous and resuscitative equipment and well-trained physicians must be in attendance throughout the procedure. Patients, who develop symptoms consistent with an IgE-mediated reaction during the graded challenge, should not receive further drug. The drug should be avoided or administered via desensitization.
Desensitization is defined as the conversion of a patient with a drug allergy from a highly sensitive state to a clinically tolerable state.94 The procedure temporarily modifies a patient's response to a drug, allowing safe treatment. Tolerance is maintained only as long as the patient continues to take the specific drug. In most instances, the patient will remain desensitized for a minimum of 24 to 48 hours, so missing a single dose does not usually necessitate to repeat desensitization. If the drug is discontinued longer, additional desensitization will be needed.7,16 Desensitization is performed by the cautious administration of incremental doses of the drug to the patient. A typical starting dose is often 1/10,000th of the final dose or twice the dose used in the skin testing,44 followed by doubling of previous dose at regular intervals until the final therapeutic dose is achieved. The length of the procedure depends on the drug and route of administration. The procedure should be performed by an allergist in a closely monitored setting.
Desensitization can be implemented in patients with known or presumed IgE antibodies to a particular drug when no alternative drug is available. Specifically, a desensitization protocol should be performed in a cephalosporin-allergic patient requiring the culprit or another cephalosporin to which there is evidence of IgE-mediated allergy. Cephalosporin desensitization should also be considered in a skin test-positive, penicillin-allergic patient requiring a cephalosporin to which the patient is skin test-positive. Similarly, penicillin desensitization should be considered in a cephalosporin-allergic patient requiring a penicillin to which the patient is skin-test positive.
In the past, drug desensitization was considered an approach to the acute management of IgE sensitivity only. However, modified forms of desensitization can be used to manage drug-induced reactions that are thought to be immunologic in nature but that are not IgE-mediated. A more prolonged, slow type of desensitization has been reported to be successful in AIDS patients with drug allergy.95 This procedure is performed over several days. The term induction of drug tolerance encompasses both IgE-mediated desensitization, as well as non-IgE-mediated mechanisms, and has replaced the term drug desensitization.23
Both graded challenge and induction of drug tolerance are contraindicated in patients with a suspected history of Stevens-Johnson syndrome, toxic epidermal necrolysis, or exfoliated dermatitis, because even small doses of the drug may induce potentially fatal recurrent desquamative reactions. Also, neither procedure should be attempted in patients with a history of severe non-IgE-mediated, immunologic reactions, such as serum sickness reactions, nephritis, hepatitis, or hemolytic anemia.23
The allergenic determinants of cephalosporins have not been fully elucidated, and the positive and negative predictive values of cephalosporin skin testing have not been fully established. Therefore, cephalosporin skin testing is not as well validated as penicillin skin testing. Despite these limitations, skin testing to penicillin and to the cephalosporin in question assists in selection of the appropriate management strategy. A positive skin test to the cephalosporin in question suggests the possible presence of drug-specific IgE antibodies, and thus, avoidance of the cephalosporin or induction of drug tolerance would be recommended if no alternative drugs were available. On the other hand, a negative cephalosporin skin test should not be interpreted as proof that an allergy is not present. In this instance, performing a graded challenge to the cephalosporin in question would be advisable; otherwise an alternative cephalosporin that has a dissimilar side chain should be considered.
Figures and Tables
 | Fig. 3Use of penicillin in patients with cephalosporin allergy. Penicillin skin testing should include both major (Pre-Pen) and minor determinant reagents (MDM). When these reagents are not available, it is advisable to select a penicillin that have a dissimilar side chain to the culprit cephalosporin, and skin testing with that penicillin in its native form. If the results are negative, the patient may be treated with a graded challenge to the penicillin. |
 | Fig. 4Use of a cephalosporin in patients with cephalosporin allergy. A desensitization protocol should be performed in patients who require the culprit cephalosporin to which there is evidence of IgE-mediated allergy. |
Table 1

Table 2

| Groups with a similar R2-side chain | |||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | ||||
| Cefdinir | Ceftazidime | ||||
| Cefixime | Cefsulodin | ||||
References
1. Norrby SR. Side effects of cephalosporins. Drugs. 1987; 34:Suppl 2. 105–120.
2. Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med. 2001; 345:804–809.
3. Romano A, Torres MJ, Namour F, Mayorga C, Artesani MC, Venuti A, et al. Immediate hypersensitivity to cephalosporins. Allergy. 2002; 57:Suppl 72. 52–57.
4. Madaan A, Li JT. Cephalosporin allergy. Immunol Allergy Clin North Am. 2004; 24:463–476. vi–vii.
5. Guéant JL, Guéant-Rodriguez RM, Viola M, Valluzzi RL, Romano A. IgE-mediated hypersensitivity to cephalosporins. Curr Pharm Des. 2006; 12:3335–3345.
6. Antunez C, Blanca-Lopez N, Torres MJ, Mayorga C, Perez-Inestrosa E, Montañez MI, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol. 2006; 117:404–410.
7. Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013; 45:131–142.
8. Moreno E, Macías E, Dávila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf. 2008; 7:295–304.
9. Sánchez-Sancho F, Perez-Inestrosa E, Suau R, Montañez MI, Mayorga C, Torres MJ, et al. Synthesis, characterization and immunochemical evaluation of cephalosporin antigenic determinants. J Mol Recognit. 2003; 16:148–156.
10. Moreno E, Dávila I, Laffond E, Macías E, Isidoro M, Ruiz A, et al. Selective immediate hypersensitivity to cefepime. J Investig Allergol Clin Immunol. 2007; 17:52–54.
11. Sáenz de San Pedro B, Mayorga C, Torres MJ, Florido JF, Quiralte J, Blanca M. Boosted IgE response after anaphylaxis reaction to cefuroxime with cross-reactivity with cefotaxime. Ann Allergy Asthma Immunol. 2002; 89:101–103.
12. Venemalm L. Pyrazinone conjugates as potential cephalosporin allergens. Bioorg Med Chem Lett. 2001; 11:1869–1870.
13. Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med. 1987; 107:204–215.
14. Macy E, Poon K-Y T. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009; 122:778.e1–778.e7.
15. Lee CE, Zembower TR, Fotis MA, Postelnick MJ, Greenberger PA, Peterson LR, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000; 160:2819–2822.
16. Lagacé-Wiens P, Rubinstein E. Adverse reactions to β-lactam antimicrobials. Expert Opin Drug Saf. 2012; 11:381–399.
17. Saxon A, Adelman DC, Patel A, Hajdu R, Calandra GB. Imipenem cross-reactivity with penicillin in humans. J Allergy Clin Immunol. 1988; 82:213–217.
18. Gell PGH, Harrington CR, Michel R. The antigenic function of simple chemical compounds: correlation of antigenicity with chemical reactivity. Br J Exp Pathol. 1948; 29:578–589.
19. Pirmohamed M, Madden S, Park BK. Idiosyncratic drug reactions. Metabolic bioactivation as a pathogenic mechanism. Clin Pharmacokinet. 1996; 31:215–230.
20. Levine BB, Ovary Z. Studies on the mechanism of the formation of the penicillin antigen. III. The N-(D-alpha-benzylpenicilloyl) group as an antigenic determinant responsible for hypersensitivity to penicillin G. J Exp Med. 1961; 114:875–904.
21. Parker CW, Deweck AL, Kern M, Eisen HN. The preparation and some properties of penicillenic acid derivatives relevant to penicillin hypersensitivity. J Exp Med. 1962; 115:803–819.
22. Perez-Inestrosa E, Suau R, Montañez MI, Rodriguez R, Mayorga C, Torres MJ, et al. Cephalosporin chemical reactivity and its immunological implications. Curr Opin Allergy Clin Immunol. 2005; 5:323–330.
23. Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010; 105:259–273.
24. Levine BB, Redmond AP. Minor haptenic determinant-specific reagins of penicillin hypersensitivity in man. Int Arch Allergy Appl Immunol. 1969; 35:445–455.
25. Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981; 68:171–180.
26. Green GR, Rosenblum A. Report of the penicillin study group-American Academy of Allergy. J Allergy Clin Immunol. 1971; 48:331–343.
27. Harle DG, Baldo BA. Drugs as allergens: an immunoassay for detecting IgE antibodies to cephalosporins. Int Arch Allergy Appl Immunol. 1990; 92:439–444.
28. Pham NH, Baldo BA. beta-Lactam drug allergens: fine structural recognition patterns of cephalosporin-reactive IgE antibodies. J Mol Recognit. 1996; 9:287–296.
29. Zhao Z, Baldo BA, Rimmer J. beta-Lactam allergenic determinants: fine structural recognition of a cross-reacting determinant on benzylpenicillin and cephalothin. Clin Exp Allergy. 2002; 32:1644–1650.
30. Assem ES, Vickers MR. Tests for penicillin allergy in man. II. The immunological cross-reaction between penicillins and cephalosporins. Immunology. 1974; 27:255–269.
31. Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother. 1975; 1:107–118.
32. Petz LD. Immunologic reactions of humans to cephalosporins. Postgrad Med J. 1971; 47:Suppl. 64–69.
33. Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis. 1978; 137:Suppl. S74–S79.
34. Romano A, Guéant-Rodriguez RM, Viola M, Pettinato R, Guéant JL. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004; 141:16–22.
35. Macy E, Mangat R, Burchette RJ. Penicillin skin testing in advance of need: multiyear follow-up in 568 test result-negative subjects exposed to oral penicillins. J Allergy Clin Immunol. 2003; 111:1111–1115.
36. Pichichero ME, Pichichero DM. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. J Pediatr. 1998; 132:137–143.
37. Pedersen-Bjergaard J. Cephalothin in the treatment of penicillin sensitive patients. Acta Allergol. 1967; 22:299–306.
38. Sullivan TJ, Ong RC, Gilliam LK. Studies of the multiple drug allergy syndrome. J Allergy Clin Immunol. 1989; 83:270.
39. Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngol Head Neck Surg. 2007; 136:340–347.
40. Audicana M, Bernaola G, Urrutia I, Echechipia S, Gastaminza G, Muñoz D, et al. Allergic reactions to betalactams: studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporin. Allergy. 1994; 49:108–113.
41. Blanca M, Fernandez J, Miranda A, Terrados S, Torres MJ, Vega JM, et al. Cross-reactivity between penicillins and cephalosporins: clinical and immunologic studies. J Allergy Clin Immunol. 1989; 83:381–385.
42. Miranda A, Blanca M, Vega JM, Moreno F, Carmona MJ, García JJ, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol. 1996; 98:671–677.
43. Sastre J, Quijano LD, Novalbos A, Hernandez G, Cuesta J, de las Heras M, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy. 1996; 51:383–386.
44. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010; 125:S126–S137.
45. Novalbos A, Sastre J, Cuesta J, De Las Heras M, Lluch-Bernal M, Bombín C, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001; 31:438–443.
46. Romano A, Guéant-Rodriguez RM, Viola M, Amoghly F, Gaeta F, Nicolas JP, et al. Diagnosing immediate reactions to cephalosporins. Clin Exp Allergy. 2005; 35:1234–1242.
47. Romano A, Quaratino D, Venemalm L, Torres MJ, Venuti A, Blanca M. A case of IgE-mediated hypersensitivity to ceftriaxone. J Allergy Clin Immunol. 1999; 104:1113–1114.
48. Orhan F, Odemis E, Yaris N, Okten A, Erduran E, Durmaz M, et al. A case of IgE-mediated hypersensitivity to cefepime. Allergy. 2004; 59:239–241.
49. Romano A, Viola M, Guéant-Rodriguez RM, Valluzzi RL, Guéant JL. Selective immediate hypersensitivity to cefodizime. Allergy. 2005; 60:1545–1546.
50. Hasdenteufel F, Luyasu S, Renaudin JM, Trechot P, Kanny G. Anaphylactic shock associated with cefuroxime axetil: structure-activity relationships. Ann Pharmacother. 2007; 41:1069–1072.
51. Romano A, Mayorga C, Torres MJ, Artesani MC, Suau R, Sánchez F, et al. Immediate allergic reactions to cephalosporins: cross-reactivity and selective responses. J Allergy Clin Immunol. 2000; 106:1177–1183.
52. Adkinson NF Jr. Immunogenicity and cross-allergenicity of aztreonam. Am J Med. 1990; 88:12S–15S.
53. Saxon A, Hassner A, Swabb EA, Wheeler B, Adkinson NF Jr. Lack of cross-reactivity between aztreonam, a monobactam antibiotic, and penicillin in penicillin-allergic subjects. J Infect Dis. 1984; 149:16–22.
54. Pérez Pimiento A, Gómez Martínez M, Mínguez Mena A, Trampal González A, de Paz Arranz S, Rodríguez Mosquera M. Aztreonam and ceftazidime: evidence of in vivo cross allergenicity. Allergy. 1998; 53:624–625.
55. Soto Alvarez J, Sacristán del Castillo JA, Sampedro García I, Alsar Ortiz MJ. Immediate hypersensitivity to aztreonam. Lancet. 1990; 335:1094.
56. Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010; 126:994–999.
57. Moss RB. Sensitization to aztreonam and cross-reactivity with other beta-lactam antibiotics in high-risk patients with cystic fibrosis. J Allergy Clin Immunol. 1991; 87:78–88.
58. Iglesias Cadarso A, Sáez Jiménez SA, Vidal Pan C, Rodriguez Mosquera M. Aztreonam-induced anaphylaxis. Lancet. 1990; 336:746–747.
59. Demoly P, Messaad D, Sahla H, Hillaire-Buys D, Bousquet J. Immediate hypersensitivity to ceftriaxone. Allergy. 2000; 55:418–419.
60. Igea JM, Fraj J, Davila I, Cuevas M, Cuesta J, Hinojosa M. Allergy to cefazolin: study of in vivo cross reactivity with other betalactams. Ann Allergy. 1992; 68:515–519.
61. Gaig P, San Miguel MM, Enrique E, García-Ortega P. Selective type-1 hypersensitivity to cefixime. Allergy. 1999; 54:901–902.
62. Pipet A, Veyrac G, Wessel F, Jolliet P, Magnan A, Demoly P, et al. A statement on cefazolin immediate hypersensitivity: data from a large database, and focus on the cross-reactivities. Clin Exp Allergy. 2011; 41:1602–1608.
63. Smith JW, Johnson JE, Cluff LE. Studies on the epidemiology of adverse drug reactions. II. An evaluation of penicillin allergy. N Engl J Med. 1966; 274:998–1002.
64. Pumphrey RS, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet. 1999; 353:1157–1158.
65. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol. 2003; 112:629–630.
66. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013; 68:702–712.
67. Romano A, Gaeta F, Valluzzi RL, Caruso C, Alonzi C, Viola M, et al. Diagnosing nonimmediate reactions to cephalosporins. J Allergy Clin Immunol. 2012; 129:1166–1169.
68. Testi S, Severino M, Iorno ML, Capretti S, Ermini G, Macchia D, et al. Nonirritating concentration for skin testing with cephalosporins. J Investig Allergol Clin Immunol. 2010; 20:171–172.
69. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002; 57:45–51.
70. Romano A, Gaeta F, Valluzzi RL, Alonzi C, Viola M, et al. Diagnosing hypersensitivity reactions to cephalosporins in children. Pediatrics. 2008; 122:521–527.
71. Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993; 270:2456–2463.
72. Sogn DD, Evans R 3rd, Shepherd GM, Casale TB, Condemi J, Greenberger PA, et al. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992; 152:1025–1032.
73. Yoon SY, Park SY, Kim S, Lee T, Lee YS, Kwon HS, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy. 2013; 68:938–944.
74. Kim JE, Kim SH, Jin HJ, Hwang EK, Kim JH, Ye YM, et al. IgE sensitization to cephalosporins in health care workers. Allergy Asthma Immunol Res. 2012; 4:85–91.
75. Fontaine C, Mayorga C, Bousquet PJ, Arnoux B, Torres MJ, Blanca M, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy. 2007; 62:47–52.
76. Kraft D, Roth A, Mischer P, Pichler H, Ebner H. Specific and total serum IgE measurements in the diagnosis of penicillin allergy. A long term follow-up study. Clin Allergy. 1977; 7:21–28.
77. Blanca M, Torres MJ, García JJ, Romano A, Mayorga C, de Ramon E, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999; 103:918–924.
78. Romano A, Gaeta F, Valluzzi RL, Zaffiro A, Caruso C, Quaratino D. Natural evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity to cephalosporins. Allergy. 2014; 69:806–809.
79. Makhija M, O'Gorman MR. Chapter 31: Common in vitro tests for allergy and immunology. Allergy Asthma Proc. 2012; 33:Suppl 1. S108–S111.
80. Sanz ML, Gamboa PM, Mayorga C. Basophil activation tests in the evaluation of immediate drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2009; 9:298–304.
81. Sanz ML, Gamboa PM, Antépara I, Uasuf C, Vila L, Garcia-Avilés C, Chazot M, De Weck AL. Flow cytometric basophil activation test by detection of CD63 expression in patients with immediate-type reactions to betalactam antibiotics. Clin Exp Allergy. 2002; 32:277–286.
82. Abuaf N, Rostane H, Rajoely B, Gaouar H, Autegarden JE, Leynadier F, et al. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin Exp Allergy. 2008; 38:921–928.
83. Leysen J, Sabato V, Verweij MM, De Knop KJ, Bridts CH, De Clerck LS, et al. The basophil activation test in the diagnosis of immediate drug hypersensitivity. Expert Rev Clin Immunol. 2011; 7:349–355.
84. Gómez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012; 4:251–263.
85. Torres MJ, Padial A, Mayorga C, Fernández T, Sanchez-Sabate E, Cornejo-García JA, et al. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin Exp Allergy. 2004; 34:1768–1775.
86. Gamboa PM, García-Avilés MC, Urrutia I, Antépara I, Esparza R, Sanz ML. Basophil activation and sulfidoleukotriene production in patients with immediate allergy to betalactam antibiotics and negative skin tests. J Investig Allergol Clin Immunol. 2004; 14:278–283.
87. Finke SR, Grieco MH, Connell JT, Smith EC, Sherman WB. Results of comparative skin tests with penicilloyl-polylysine and penicillin in patients with penicillin allergy. Am J Med. 1965; 38:71–82.
88. Hoffman DR, Hudson P, Carlyle SJ, Massello W 3rd. Three cases of fatal anaphylaxis to antibiotics in patients with prior histories of allergy to the drug. Ann Allergy. 1989; 62:91–93.
89. Spruill FG, Minette LJ, Sturner WQ. Two surgical deaths associated with cephalothin. JAMA. 1974; 229:440–441.
90. Thong BY. Update on the management of antibiotic allergy. Allergy Asthma Immunol Res. 2010; 2:77–86.
91. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009; 64:183–193.
92. Torres MJ, Blanca M, Fernandez J, Romano A, Weck A, Aberer W, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003; 58:961–972.
93. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–863.
94. Gruchalla R. Understanding drug allergies. J Allergy Clin Immunol. 2000; 105:S637–S644.
95. Naranjo CA, Shear NH, Lanctôt KL. Advances in the diagnosis of adverse drug reactions. J Clin Pharmacol. 1992; 32:897–904.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download