Abstract
Glatiramer acetate (GA) is a synthetic amino acid polymer, used for relapsing-remitting multiple sclerosis. The most common adverse effect of GA is a skin reaction at the injection site with a probable IgE-mediated mechanism. We report a case of a 45-year-old woman with multiple sclerosis and urticaria to interferon-β1a, who underwent a challenge test to GA. She presented itching wheals at the intradermal sites. A month later the patient repeated the test and presented the same reactions of the first test. The next day she continued the test with subcutaneous injections. One hour later she presented a flare up of the reactions appeared during the previous 2 tests. No reactions appeared at the subcutaneous injection sites. The patient also presented dyspnea. Flare-up reactions are characterized by the reactivation of previously positive reactions to intradermal or skin tests triggered by patch testing and after systemic provocation with an allergen. The phenomenon is not common to drugs. The mechanisms involved in this reaction seem to be heterogeneous and are not completely understood. To our knowledge this is the first case of allergic reaction to GA manifested as a flare-up reaction during challenge test.
GA is a synthetic amino acid polymer composed of a mixture of L-glutamic acid, L-lysine, L-alanine, and L-tyrosine in defined proportions, used for immunomodulatory therapy of relapsing-remitting multiple sclerosis (RRMS). GA has been found to alter the natural history of the disease by reducing the relapse rate and affecting disability.
Generally, it is viewed that GA has the most favourable adverse effect profile compared with the other therapeutic options available for MS. However, approximately 15% of patients experience a self-limited, postinjection systemic reaction characterized by chest tightness, flushing, anxiety, dyspnea, and palpitations. This reaction is unpredictable, can occur at any time during treatment, and may be mistaken for cardiac ischemia.1 The most common adverse effect of GA is a skin reaction at the injection site.2,3 In this immediate-type local reaction an IgE-mediated mechanism is probably involved.2
It is also known that GA can cause anaphylactic reactions with different grade of severity, varying from generalized pruritic exanthema over the whole body, to more serious reactions as bronchial spasm and shock with severe hypotension and loss of consciousness.3,4 A case of contact dermatitis with a positive lymphocyte transformation test has been also reported.5
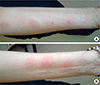
A 45-year-old woman with a 3-year history of RRMS and no history of adverse drug reactions, experienced an urticarial reaction to interferon-β1a three month after beginning the therapy. She referred to our allergy department to test GA as an alternative drug. Skin prick and intradermal tests were performed on the volar side of the left forearm using commercial GA (20 mg/mL). Skin prick test was negative. The intradermal test, performed with scalar dilutions to 1:100, 1:10, 1:1 with reading at 20 minutes was positive. Indeed the patient presented a 20-30 mm itching wheal at all the 3 intradermal sites. Therefore we decided to stop the test without performing the intradermal test with undiluted drug and the challenge test with subcutaneous injections. A month later the patient again underwent an intradermal test to GA diluted to 1:100, 1:10, 1:1 and undiluted on the volar side of the right forearm. She presented the same reaction of the first test at the injection sites at all the dilutions and at the undiluted injection. The next day the skin reactions were decreased. Therefore we continued the test with 2 GA subcutaneous injections of 0.4 mL and 0.6 mL on the right and left arm respectively. The time interval between the 2 injections was of an hour and half. One hour after the last injection the patient presented itching wheals on the right forearm at the sites where intradermal tests had been performed the day before at the dilutions 1:100, 1:1 and undiluted (Figure A). At the same time, she presented other three itching wheals on the left forearm, at the same sites of the intradermal tests conducted the previous month (Figure B). No reactions were present at the sites of subcutaneous injections. Thirty minutes later the patient presented shortness of breath and she was treated with betamethasone 4 mg/day for 3 days.
Two months later the patient underwent a challenge test for azathioprine without any adverse reaction.
The patient presented an urticarial reaction during interferon-β1 a therapy. However, we did not perform a provocation test with this drug because in our clinical practice, for patient's safety, it is not common to perform a provocation test with the same drug that has caused the reaction, except when it is not available an alternative molecule. Being GA the alternative drug suggested by the patient's neurologist, we performed the challenge test for this molecule.
Flare-up reactions are characterized by the reactivation of previously positive reactions to intradermal or skin tests triggered by patch testing and after systemic provocation with an allergen. The phenomenon has most frequently been described with nickel.6
Flare-up reactions to drugs are not common. The literature reports a few cases of flare-up reactions to antibiotics as betalactams7 and neomycin,8 to heparin9 and to gold sodium thiomalate.10
The mechanisms involved in this reaction seem to be heterogeneous and are not completely understood.
Some of the reported flare-up reactions have been clearly classified as delayed-type hypersensitive reactions, since they appeared 12 to 24 hours after the challenge-test.7,9 In our patients, indeed, the intradermal reactions appeared in a very short time (20 minutes) and the reactivation of the previous intradermal reactions appeared one hour later the subcutaneous administration, the necessary time to a drug to reach a systemic diffusion. Therefore, it is possible to hypothesize the involvement of an immediate mechanism.
Local injection site reactions to GA are frequent. However, the reactions presented by the patient could not be considered as the known skin reaction after GA injections. In fact, the patient did not present any reaction after subcutaneous injections during challenge-test and reported the wheals accompanied by intense itch. We do not know the reason of the absence of reaction at the subcutaneous sites; it is possible to hypothesize a memory mechanism involving skin mast cells. Two other patients who underwent a challenge test to GA for a previous allergy to interferon did not present any reaction at the injection sites of intradermal tests.
Furthermore, since GA is an amino acid polymer, it is not easy to identify the antigen involved; however, the formulation of GA contains mannitol, which has previously been involved in IgE-mediated adverse reactions. Therefore, mannitol could be a plausible antigen in allergic reactions to formulation of GA. However, in the published studies on GA allergic reactions is not reported a specific antigen involved.
To our knowledge this is the first case of allergic reaction to GA manifested as a flare-up reaction during challenge test. This case suggests that a flare up phenomenon could represent a rare type of adverse reaction to GA during a provocation test.
In conclusion, during a challenge test to GA flare-up reactions should be taken into account on the strength to the history of the patient.
Figures and Tables
References
1. Ruggieri M, Avolio C, Livrea P, Trojano M. Glatiramer acetate in multiple sclerosis: a review. CNS Drug Rev. 2007; 13:178–191.
2. Sánchez-López J, Rodríguez del Rio P, Cases-Ortega B, Martínez-Cócera C, Fernández-Rivas M. Allergy workup in immediate-type local reactions to glatiramer acetate. J Investig Allergol Clin Immunol. 2010; 20:521–523.
3. Baumgartner A, Stich O, Rauer S. Anaphylactic reaction after injection of glatiramer acetate (Copaxone®) in patients with relapsing-remitting multiple sclerosis. Eur Neurol. 2011; 66:368–370.
4. Soriano Gomis V, Pérez Sempere A, González Delgado P, Sempere JM, Niveiro Hernández E, Marco FM. Glatiramer acetate anaphylaxis: detection of antibodies and basophil activation test. J Investig Allergol Clin Immunol. 2012; 22:65–66.
5. Haltmeier S, Yildiz M, Müller S, Anliker MD, Heinzerling L. Contact dermatitis induced by glatiramer acetate. Mult Scler. 2011; 17:1390–1392.
6. Boscolo P, Andreassi M, Sabbioni E, Reale M, Conti P, Amerio P, Di Gioacchino M. Systemic effects of ingested nickel on the immune system of nickel-sensitized women. Life Sci. 1999; 64:1485–1491.
7. Reig Rincón de Arellano I, Villalón García AL, Cimarra Alvarez-Lovell M, Robledo Echarren T, Martínez-Cócera MC. Flare up to betalactams. Allergol Immunopathol (Madr). 2005; 33:282–284.
8. Jacob SE, Barland C, ElSaie ML. Patch-test-induced "flare-up" reactions to neomycin at prior biopsy sites. Dermatitis. 2008; 19:E46–E48.
9. García Robaina JC, Sánchez Machín I, Fernández-Caldas E, de la Torre Morín F. Delayed systemic reactions with flare-ups of previously negative intradermal skin tests to heparin. Allergy. 2003; 58:685–686.
10. Möller H, Ohlsson K, Linder C, Björkner B, Bruze M. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999; 40:200–204.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download