Abstract
Purpose
Fixed drug eruption (FDE) is characterized by a well-defined erythematous patch, plaque, or bullous eruption that recurs at the same site as the result of systemic exposure to a causative drug, and resolves with or without hyperpigmentation. This study was carried out to identify the common causative drugs and clinical features of FDE in Korea.
Methods
We reviewed electronic medical records of all patients diagnosed with FDE from January 2000 to December 2010 at a tertiary hospital in Korea.
Results
A total of 134 cases were diagnosed as FDE. The mean age was 35.9 years (range, 0-82 years) and 69 (51.5%) of the patients were male. The mean duration from the first event to attending hospital was 1.9 years (range, 1-20 years). The mean number of recurrences was 2.6 (1-10), and 72.6% of patients sought medical care after experiencing symptoms twice or more. Four patients (3.1%) needed hospitalization. The most common sites were the upper extremities (47.7%), followed by the lower extremities, face, abdomen, chest, buttocks and perineum. Clear documentation on the causative drugs was available for 38 patients (28.4%), and among these, non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen accounted for 71.1% of cases, and antibiotics accounted for 15.8%. Eighty patients (59.7%) underwent active treatment for FDE, and topical steroids were most frequently prescribed (43.3%), with systemic steroids used in 11.2% of patients.
Drug eruption refers to an unexpected cutaneous lesion that occurs after a specific drug is administered and is known to be the cause of ~2%-3% of dermatological problems.1 Maculopapular eruption is the most common type of drug eruption, but it is difficult for physicians to diagnose and differentiate between rashes related to infectious conditions. Although fixed drug eruption (FDE) is less common, its diagnosis is usually straightforward.
FDE was first reported in 1889 by Bourns, when he described a lesion that repeatedly developed at the same limited sites after antipyrine was administered.2 In 1894 Brocq named this type of lesion an "eruptio-erythemato-pigmentee fixe".3 FDE is currently defined as a cutaneous adverse drug reaction in which a lesion recurs on the same site on the skin or mucous membrane, whenever the causative drug is re-administered. The skin lesion disappears when medication is discontinued, but it can sometimes result in permanent pigmentation.4,5,6 Because of its characteristic features, FDE can be diagnosed with relative ease compared to other drug eruptions.
The number of diagnosed FDE cases is increasing steadily, due in part to increased awareness by physicians as well as increased requests by patients to identify the precise cause of repeated eruptions and pigmentation.7 Recently, awareness of adverse drug reactions has increased, but no large-scale study has been carried out to provide objective information on the clinical aspects of FDE in Korea.
This study therefore aimed to analyze cases of FDE at a tertiary medical institution and to identify common causative drugs as well as the clinical patterns of FDE in Korea.
Patients diagnosed with FDE at the Seoul National University Hospital between January 2000 and December 2010, were reviewed retrospectively as follows: A) data were obtained from the medical records of patients diagnosed with FDE, B) 2 allergy specialists validated the cases if they satisfied at least one of the following conditions; 1) lesion(s) of the same form occurring twice or more at the same site as a result of a re-administration of a causative drug; 2) confirmation by a challenge test; and 3) the typical morphological features compatible with FDE were alleviated by discontinuation of the causative drug, and the possibility of other diagnoses was low.4 Cases were excluded if biopsy findings suggested other diagnoses. The causal relationship was evaluated using the WHO-UMC causality assessment system and cases with 'certain' or 'probable' causality were included in the analysis.
We analyzed the age, gender, causative drugs, and morphological features of lesions, onset, treatment, and recurrence of symptoms.
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as means and standard deviations (range). Groups were compared by Fischer's exact test, a Chi-squared for trend, and a t-test. Statistical significance was accepted at P<0.05.
A total of 184 patients were diagnosed with FDE between 2000 and 2010. Among them, 50 were excluded after clinical history reviews, leaving a total of 134 patients in the study (Table 1). 97 patients (72.6%) experienced recurrent FDE with a causative drug and 9 patients (6.7%) were diagnosed with FDE by a challenge test. Among the study subjects, 69 (51.5%) were male; the mean age was 35.9 years with a range of 0 to 82 years.
The mean duration from the onset of FDE to the first hospital visit was 1.9 years (0-20 years). Sixty-six patients (49.3%) had typical lesions compatible with FDE at admission, which improved with discontinuation of the causative drug. While typical bullous lesions of active FDE were found in 36.5% of the subjects, 68 (56.7%) had only remnant pigmentation when they saw the physician. Only 27.4% visited the hospital at the onset of lesion development, and 72.6% sought medical care after experiencing repeated symptoms. On average, patients visited the hospital after experiencing FDE 2.6 times.
Although there were 2 cases of generalized bullous FDEs, there were no fatalities. One patient had recurrent skin reactions after administration of acetaminophen and another patient experienced a first episode of FDE after taking mefenamic acid. They were diagnosed with generalized bullous FDEs because of the temporal correlation with the drug, the well-demarcated erythematous macules and bullae on the trunk, face and extremities without other systemic symptoms, and the rapid recovery that left residual hyperpigmentation after discontinuation of causality drugs.
Most of the subjects (97%) were treated for FDE in outpatient clinics. Among the 4 hospitalized patients, 2 were admitted for care of generalized bullous FDE and 1 for management of multiple bullous lesions in the mouth, hands and penis, accompanied by severe pain and fever. The fourth patient had skin exfoliation as well as typical bullous lesions of FDE and was admitted for close observation and to determine whether or not the symptoms were due to FDE or Stevens-Johnson syndrome (SJS).
Among 52 patients with active lesion(s) during their visit to the hospital, bullous lesions were observed in 19 (36.5%). Skin biopsies were carried out in 17 patients (12.7%) to differentiate FDE from other diseases.
The most common site of FDE was the upper limbs (47.7%) followed by lower limbs (29.9%), face (24.6%), abdomen (17.6%), chest (17.2%), and back (16.4%). FDE lesions were not always localized, and in 41 patients (30.6%) they were widely distributed on the body (Fig. 1A). FDEs frequently developed as multiple lesions but a single solitary lesion was observed in 30.6% of FDE cases (Fig. 1B). Frequently involved sites differed slightly depending on the number of lesions. When FDE appeared at multiple sites, the upper limbs were affected most frequently, followed by the lower limbs, face, abdomen, and chest (Fig. 1C). When FDE developed as a single solitary lesion, the upper limbs were still affected (34.1%), but the lower limbs were less affected (4.9%) (Fig. 1C).
In most cases, the link between the causative drug and FDE was determined by a recurrence of symptoms through re-challenge with the drug. In 62 subjects (46.3%), the class or category of causative drug was described in their medical records: cold remedy (26), analgesics (12), antibiotics (10) and others (14). Accurate causative drugs were accessed only in 38 patients (28.4%). Non-steroidal anti-inflammatory drugs (NSAIDs) were the most common drugs (71.1%) causative to FDE. Acetaminophen was a single drug, which was most frequently implicated in FDE (23.7%) (Table 2). A further analysis of areas affected on the body revealed that FDE, resulting from chemotherapeutic agents, was found more frequently in the perineum compared to other drugs (66.7% vs 12.1%, P=0.015). However, the number of lesions was not related to the causative agents. A description of the causative agents was lacking in 34 cases (25.4%) (Fig. 2).
It was not possible to compare the severity of FDE with specific causative agents. However, most of the patients with NSAIDs-induced FDE (81.5%) sought doctors when they experienced repeated FDE, while patients with FDE induced by other drugs tended to visit the hospital when they experienced FDE for the first time (60%).
After a diagnosis of FDE, 54 patients (40.3%) did not receive any treatment, and 58 patients (43.3%) used a topical steroid (Fig. 3). A local treatment other than topical steroid was prescribed for 26 patients (19.4%) and antihistamines were prescribed for 19 patients (14.2%). A systemic steroid equivalent to prednisolone (26.0±19.2 mg) was prescribed for 15 patients (11.2%) for an average of 11.5±9.7 days. Patients with acute lesions were prescribed systemic steroids in 23.1% cases. Systemic steroid use was not related to any causative drugs or to a multiplicity of FDE lesions.
FDEs develop as round, or oval erythematous plaques with discrete margins, in single or multiple lesions, and can occur as bullae in severe cases. With repeated FDE occurrences, the number and size of lesions tends to increase and they become darker in color. FDE usually develops at 0.5-8 hours after administration of a causative drug, with a mean onset time of 2 hours.4 The FDE lesions usually disappear ~3 weeks after discontinuation of the causative medication, but sometimes cause skin pigmentation.4 In this study, over half of the patients exhibited pigmentation only at their first hospital visit, and this was ~2 years after their initial FDE symptoms.
Acute FDE lesions mainly appear as erythematous or colored spots, with blisters or bullous lesions developing in some instances. Occasionally they appear in the form of erosion or necrosis around an ulcer, and this is frequently observed in perineal lesions.8 In this study, ~43.3% of the patients had acute lesions during their hospital visit; 36.5% of these were bullous lesions. These findings are similar to the results of a previous study.9
Generalized bullous FDE is a relatively severe reaction that should be differentiated from SJS or toxic epidermal necrolysis (TEN). Generalized bullous FDE usually develops as multiple deep scarlet papules or bullous lesions with discrete margins that are distributed symmetrically on the entire body. The size or number of lesions can increase even after the withdrawal of the causative drugs. Generalized bullous FDE is known to be related to a severe reaction to repeated exposure to causative agents in patients who have already had FDE in the past.10 In this study, generalized bullous FDE was found in 1.5% of the total cases, and none were fatal. FDE (and generalized bullous FDE) seems to be a relatively mild drug eruption in contrast to other bullous drug eruptions, such as SJS or TEN; this finding is consistent with previous studies.10
FDE can develop on any part of the skin or mucous membrane. The most common sites involved are the trunk, limbs, lips, palms, soles, penis, and groin.5 Sharma et al.11 reported that frequently involved sites varied according to the causative drug; tetracycline induced FDE in the mucocutaneous junction of the genital organs, metamizole sodium (analgin®) induced lesions on the trunk and limbs, and phenytoin sodium was significantly associated with generalized FDE. In our study, these associations were not observed; however, we did note that FDE related to chemotherapeutic agents occurred more frequently in the perineal area. As well as the distribution of lesions, some studies reported an association between multiplicity and specific drugs. Tetracycline, trimethoprim-sulfamethoxazole, and butazolidine were reported to be linked with single solitary lesions,11,12 and ibuprofen, trimethoprim-sulfamethoxazole, theophylline, and atenolol, were reported to be linked to multiple lesions.7 These correlations were not observed in this study.
Previously reported incidences of FDE varied from 2.5%-22% in patients with dermatological problems.4,13 Several factors are known to affect FDE incidences, including geographic location, availability and drug dosage, and the age of the patient.2 Therefore, FDE incidences and causative drug patterns can vary according to the survey area and over time. Antibiotics, antipsychotics, and NSAIDs are known to be major causes of FDE. In studies performed before 2000, the most common causative agent of FDE was trimethoprim-sulfamethoxazole.14,15,16 In the past, FDEs caused by NSAIDs and acetaminophen were known to be rare,18 but in reports after 2000, NSAIDs (16%-35%) were the second most common factor after antibiotics (39%-65%), and followed by anticonvulsant drugs (16%-30%).19,20,21 In Korea, FDE caused by NSAIDs was first reported in 1996,22 and other cases have since been reported.23,24 NSAIDs and acetaminophen were the most common cause of FDE in our study, probably because they can be purchased easily without prescription. In the 1980s, pyrazolone was the most frequently reported causative agent of FDE,25,26,27 and fepraxone in 1993.28 Recently, aspirin use in children has reduced, due to risks associated with inducing Reye's syndrome. In contrast, acetaminophen use has increased in adults and children because it is relatively safer with fewer gastrointestinal side-effects.25,26,27,28,29 In a recent French study on FDE, acetaminophen was the single most common causative drug for FDE.30 Similarly, acetaminophen was found to be the single most common causative drug in this study. Since cross reactions between NSAIDs and acetaminophen were reported in FDE occurrences, patients who have previously experienced FDE after taking these drugs should be careful when choosing analgesics.31
Although clinical history is most important in diagnosing FDE, patch tests and drug challenge tests are also helpful and are used frequently for a more objective diagnostic approach. To decrease false negative responses from patch tests, the appropriate location and timing are both critical.32 Moreover, a false negative response can occur if patients are sensitized not only to the drug itself but also to its metabolites. An insufficient dose and inability of the drug to effectively infiltrate into the skin can also result in a false negative response.33 The most accurate diagnostic tool for FDE is a drug challenge test. The challenge test can be performed at varying doses, and usually starts with a very low dose that is increased gradually to a therapeutic dosage.4 A lymphocyte transformation test is a laboratory test approved for delayed drug hypersensitivities such as maculopapular eruption, drug hypersensitivity syndrome and SJS,34 but its usefulness in testing for FDE is still under investigation, even though some studies have reported positive results.18,35 These diagnostic tests are clinically important, not only for diagnosis, but also for identification of the causative drug(s).
The first step in treating FDE is identification and discontinuation of the causative drug. In many cases, discontinuation of the medication alone can improve symptoms, but antihistamines and topical steroids can be used for more immediate relief of symptoms. In the case of extensive or bullous lesions, administration of a systemic steroid is needed.7 However, it is not clear whether steroid treatment at the acute stage prevents pigmentation. In our study, almost 40.3% of patients were not prescribed any medication because more than half of them visited the hospital after their acute lesions had disappeared. Around 10% of the patients were prescribed a short-term systemic steroid, and most of these patients were at the acute stage with severe symptoms when they visited the hospital.
The most important method of preventing secondary occurrences of any adverse drug reaction is identification of the causative drug, followed by complete avoidance of repeated exposure to the causative drug. Particularly in skin allergies, repeated exposure increases the risk of recurrence, and in some cases evolves into a more severe form. Thus, it is very important to determine the specific causative drug.5,33 Moreover, a lack of information on the exact causative drug may increase the anxiety of patients about future medication, which, in turn, prevents them from obtaining adequate treatment. In our study, specific causative drugs were clearly recorded in 28.4% of the patients, whereas only the type of drug was described in more than half of the patients. Accordingly, most of the patients did not know the causative drug, and in future more effort should be made to evaluate causative agents for complete prevention of FDE.
This study was limited due to its retrospective cross-sectional nature; most diagnoses were made on the basis of typical clinical histories without confirmation by a drug challenge. Nevertheless, by examining the clinical characteristics, causative drugs, and treatment of FDE over a long period and in a large number of FDE patients in Korea, this study can serve as a reference point for clinical practice in the diagnosis and treatment of FDE.
In conclusions, FDE is a relatively common drug hypersensitivity, and over the past 11 years, 130 patients have been diagnosed with FDE at a tertiary medical institution in Korea. NSAIDs and acetaminophen were the most commonly causative drugs. Acetaminophen was the most common single causative agent of FDE in Korea. To prevent recurrence of FDE due to repeated exposure, more effort should be made to identify the causative drugs.
Figures and Tables
Fig. 1
Involved sites of patients with fixed drug eruption. (A) Most commonly involved sites were the upper extremities, followed by the lower extremities, face, abdomen, chest, buttocks, and perineum. (B) FDE cases developed as multiple lesions in 59.4% and as solitary lesion in 30.6%. (C) Involved sites differed between patients with multiple lesions and those with a solitary lesion.

Fig. 2
Causative drugs of fixed drug eruption. Causative drugs were described in broad, ambiguous terms in 46.3% of the study subjects (A) and in specific, accurate terms in 28.4% of the study subjects (B). NSAIDs, Non-steroidal anti-inflammatory drugs.
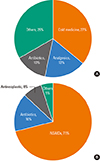
Fig. 3
Treatment for fixed drug eruption. Topical steroids were prescribed most frequently for FDE, followed by other topical agents, oral antihistamines, and systemic steroids.

Table 1
Demographics of patients with fixed drug eruption (n=134)

Table 2
Causative agents of fixed drug eruption in 38 patients whose causative drugs were accurately described in the medical charts

ACKNOWLEDGMENTS
This research was supported by a grant from Ministry of Food and Drug Safety to operation of the regional pharmacovigilance center in 2013.
References
1. Arndt KA, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976; 235:918–923.
2. Bourns DC. Unusual effects of antipyrine. Br Med J. 1889; 2:818–820.
3. Brocq L. Eruption erythémato-pigmentée fixé due à l'antipyrine. Ann Dermatol Syphiligr (Paris). 1894; 5:308–313.
4. Sehgal VN, Gangwani OP. Fixed drug eruption. Current concepts. Int J Dermatol. 1987; 26:67–74.
5. Nigen S, Knowles SR, Shear NH. Drug eruptions: approaching the diagnosis of drug-induced skin diseases. J Drugs Dermatol. 2003; 2:278–299.
6. Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987; 17:403–407.
7. Lee AY. Fixed drug eruptions. Incidence, recognition, and avoidance. Am J Clin Dermatol. 2000; 1:277–285.
8. Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006; 45:897–908.
9. Bandino JP, Wohltmann WE, Bray DW, Hoover AZ. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009; 15:4.
10. Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005; 209:123–129.
11. Sharma VK, Dhar S, Gill AN. Drug related involvement of specific sites in fixed eruptions: a statistical evaluation. J Dermatol. 1996; 23:530–534.
12. Nnoruka EN, Ikeh VO, Mbah AU. Fixed drug eruption in Nigeria. Int J Dermatol. 2006; 45:1062–1065.
13. Browne SG. Fixed eruption in deeply pigmented subjects: clinical observations on 350 patients. Br Med J. 1964; 2:1041–1044.
14. Sharma VK, Dhar S. Clinical pattern of cutaneous drug eruption among children and adolescents in north India. Pediatr Dermatol. 1995; 12:178–183.
15. Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998; 37:833–838.
16. Kanwar AJ, Bharija SC, Singh M, Belhaj MS. Ninety-eight fixed drug eruptions with provocation tests. Dermatologica. 1988; 177:274–279.
17. Alanko K, Stubb S, Kauppinen K. Cutaneous drug reactions: clinical types and causative agents. A five-year survey of in-patients (1981-1985). Acta Derm Venereol. 1989; 69:223–226.
18. Hayashi H, Shimizu T, Shimizu H. Multiple fixed drug eruption caused by acetaminophen. Clin Exp Dermatol. 2003; 28:455–456.
19. Gupta R. Drugs causing fixed drug eruptions: confirmed by provocation tests. Indian J Dermatol Venereol Leprol. 2003; 69:120–121.
20. Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol. 2004; 70:20–24.
21. Patel RM, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol Venereol Leprol. 2008; 74:430.
22. Nahm DH, Suh CH, Park HS. A case of fixed drug eruption induced by mefenamic acid. J Asthma Allergy Clin Immunol. 1996; 16:346–349.
23. Min EJ, Lim DH, Kim JH, Choi SW, Son BK. A case of fixed drug eruption due to acetaminophen. J Korean Pediatr Soc. 2000; 43:1149–1152.
24. Hong KB, Jin YM, Kang J, Im IJ, Roh EJ, Son JS, Chung EH. A case of fixed drug eruption caused by acetaminophen in a child. Pediatr Allergy Respir Dis. 2007; 17:314–319.
25. Bigby M, Stern R. Cutaneous reactions to nonsteroidal anti-inflammatory drugs. A review. J Am Acad Dermatol. 1985; 12:866–876.
26. Bailin PL, Matkaluk RM. Cutaneous reactions to rheumatological drugs. Clin Rheum Dis. 1982; 8:493–516.
27. Pasricha JS. Drugs causing fixed eruptions. Br J Dermatol. 1979; 100:183–185.
28. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. Analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993; 186:164–169.
29. Sehgal VN. Paracetamol-induced bilateral symmetric, multiple fixed drug eruption (MFDE) in a child. Pediatr Dermatol. 1999; 16:165–166.
30. Brahimi N, Routier E, Raison-Peyron N, Tronquoy AF, Pouget-Jasson C, Amarger S, Machet L, Amsler E, Claeys A, Sassolas B, Leroy D, Grange A, Dupuy A, Cordel N, Bonnetblanc JM, Milpied B, Doutre MS, Guinnepain MT, Barbaud A, Chosidow O, Roujeau JC, Lebrun-Vignes B, Descamps V. A three-year-analysis of fixed drug eruptions in hospital settings in France. Eur J Dermatol. 2010; 20:461–464.
31. Kidon MI, Kang LW, Chin CW, Hoon LS, See Y, Goh A, Lin JT, Chay OM. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to nonsteroidal antiinflammatory drugs among young, Asian, atopic children. Pediatrics. 2005; 116:e675–e680.
32. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001; 45:321–328.
33. Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009; 9:316–321.
34. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004; 59:809–820.
35. Gimenez-Camarasa JM, Garcia-Calderon P, de Moragas JM. Lymphocyte transformation test in fixed drug eruption. N Engl J Med. 1975; 292:819–821.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download