Abstract
Purpose
Sensitization to specific allergens may be important in the development of allergic airway inflammation and airway hyperresponsiveness (AHR). We evaluated the effect of specific aeroallergen sensitization on eosinophilic airway inflammation and AHR.
Methods
We reviewed retrospectively the clinical data of subjects who underwent skin prick tests to aeroallergens, induced sputum analysis, and methacholine bronchial provocation tests to evaluate lower airway symptoms as well as analyzed the associations between the pattern of aeroallergen sensitization and sputum eosinophilia or AHR.
Results
Of the 1,202 subjects be enrolled, 534 (44.4%) were sensitized to at least one aeroallergen in skin tests. AHR was demonstrated in 23.5% and sputum eosinophilia in 38.8%. Sputum eosinophilia was significantly associated with sensitization to perennial allergens (OR, 1.9; 95% CI, 1.4-2.5), house dust mite (OR, 1.7; 95% CI, 1.3-2.3), dog (OR, 1.9; 95% CI, 1.1-3.3), and cat (OR, 2.1; 95% CI, 1.4-3.4). AHR was associated with sensitization to perennial allergens (OR, 2.7; 95% CI, 2.0-3.7), house dust mite (OR, 2.2; 95% CI, 1.6 3.2), Alternaria (OR, 2.3; 95% CI, 1.2-4.7), and cat (OR, 2.7; 95% CI, 1.7-4.3). Sensitization to more perennial allergens increased the risk for sputum eosinophilia and AHR. There was no relationship with individual seasonal allergens.
Allergic diseases continue to increase in prevalence worldwide and many environmental factors contribute to their development such as allergens and exposure to air pollution.1 Sensitization to an allergen is a critical step in the induction of allergy.2,3 Allergic diseases induced by aeroallergens can show various clinical manifestations such as rhinoconjuctivitis, eosinophilic bronchitis, and asthma. Airway inflammation and airway hyperresponsiveness (AHR) are major components of asthma. Eosinophilic airway inflammation is a typical feature of asthma, although inflammation in asthma is known to be more heterogeneous.4 Eosinophilic airway inflammation can lead to the development of bronchial hyperreactivity;5 however, unlike non-asthmatic eosinophilic bronchitis, it is not always accompanied with AHR. Occasionally, some patients with allergic rhinitis may also exhibit AHR or sputum eosinophilia, even though they have no asthmatic symptoms.6,7
Prior studies have reported an association between the specific aeroallergen sensitization and the development of asthma or AHR. Sensitization to house dust mite or mold was associated with increasing asthma.8 Cockroach allergy also has been reported as an important cause of asthma.9 There was also a trend in increasing AHR with increased cat allergen exposure.10 However, sensitization to pollen was not significantly associated with the presence of asthma symptoms or AHR.11
Previous studies have generally focused on the prevalence of allergen sensitization among the general population, among patients with allergic diseases, or on the development of asthma according to sensitization to some allergens.8,12-14 Limited studies have been conducted in regards to the risk of airway inflammation and AHR according to the patterns of aeroallergen sensitization. Furthermore, allergen sensitization pattern and its influence on allergic disease may vary dependent on ethnicity and geography; however, this issue has rarely been explored in adult populations in Asia. Here, we investigated the relationship between patterns of aeroallergen sensitization and the development of eosinophilic airway inflammation and AHR in Korean adults who had allergic and lower airway symptoms.
We retrospectively reviewed the data of adult patients (age ≥ 15 years) who visited the asthma and allergy clinic at Seoul National University Bundang Hospital between January 2005 and June 2011. To investigate allergen sensitization patterns that affect airway hyperresponsiveness and airway inflammation, the clinical data were collected from patients who underwent skin prick tests to aeroallergens, a methacholine bronchial provocation test, and induced sputum analysis in the evaluation of allergic and lower airway symptoms (such as dyspnea, chronic cough, wheezing, and chest discomfort).
Exposure of the subjects to relevant allergens was assessed at the time the tests were performed. Our clinic routinely asked patients about environmental conditions and allergen exposure before tests. The presence of fabrics or carpets at home was assumed to mean that the subjects had been exposed to house dust mites and exposure to cats or dogs was considered for subjects with pets at home. Exposure to sensitized pollen was assessed when the tests were performed during the pollen season and based on the Korean calendar for allergenic pollen.15 This study was approved by the hospital institutional review board.
We analyzed the results of skin prick tests for 12 non-cross acting common inhalant aeroallergens in Korea: 6 perennial allergens that included house dust mite (D. pteronyssinus and D. farinae), Alternaria, Aspergillus, cockroach, dog, and cat, 6 seasonal allergens that included birch, elm, poplar, grass pollen mix (grasses, barley, oat, rye, and wheat), ragweed, and mugwort. Normal saline (0.9%) and histamine (1 mg/mL) were used as negative and positive controls. The skin prick test was performed after the withdrawal of antihistamines or antidepressants for at least 72 hours prior to the test. The wheal diameter was measured 15 minutes after application. Skin prick test reactions were graded by the ratio of the allergen wheal diameter to the histamine wheal diameter (A/H ratio), as grade 1+ to 4+. Grade 2+ meant an A/H ratio of ≥0.5, and each additional plus indicated a doubling of the ratio. The test was considered positive when the A/H ratio was 1 or greater. Atopy was defined as a positive skin test response to at least 1 allergen.
The patients were instructed to withhold medications (such as beta-agonists, inhaled corticosteroids, leukotriene antagonists, and antihistamines) that might interfere with the methacholine bronchial provocation test 72 hours prior to testing and to avoid caffeine on the day of testing.
A baseline spirometry was performed using a Vmax 2130 spirometer (VIASYS Respiratory Care, Inc., Yorba Linda, CA, USA). Forced expiratory volume in 1 second (FEV1) and forced vital capacity index were recorded. A bronchial provocation test was not administered for patients with FEV1 less than 70% of predicted. Methacholine dilutions of 1, 4, 16, and 25 mg/mL were used. The methacholine challenge was performed using a five-breath protocol with a dosimeter (KoKo Dosimeter; nSpire Health, Inc., Louisville, CO, USA). After 90 seconds, spirometry was performed. The test was ended when a decrease of 20% or greater compared with baseline FEV1 was achieved or when the highest concentration of methacholine was inhaled. The provocation concentration that caused a decrease of 20% in FEV1 was expressed as the PC20 (provocation concentration dose of methacholine). AHR was defined as a PC20 of less than 16 mg/mL.
Sputum induction and processing were conducted according to the previously described standardized protocol.16 Briefly, subjects inhaled nebulized 4.5% saline via an ultrasonic nebulizer (Omron NE-U17 Ultrasonic Nebulizer; Morton Medical, UK) for 5 minutes periods up to 20 minutes. Every 5 minutes after the start of nebulization, subjects spat the sputum into a Petri dish. After the addition of 0.01 M dithiothreitol, the samples were vortex-mixed, shaken for 30 minutes at room temperature, and filtered through a 100-µm mesh. The cells were collected by centrifugation (2,000 rpm, 4℃, 10 minutes) and suspended in phosphate-buffered saline to a volume equal to the original sputum plus dithiothreitol. The cells were counted using a hemocytometer, and cell concentrations were adjusted to 1.0×106 cells/mL. Cytospins were prepared by adding 60 µL of cell suspension to Shandon II cytocentrifuge cups (Shandon Southern Instruments, Sewickley, PA, USA) and were spun for 5 minutes at 42×g. The cells were stained with Diff-Quik solution (Sysmex Co., Kobe, Japan). Leukocytes, bronchial epithelial cells, and squamous cells were counted respectively. These counts were expressed as percentages of a total of 400 nucleated cells counted per slide (excluding squamous epithelial cells) to determine cell differentiation. Sputum eosinophilia was regarded as positive when the sputum eosinophil count was ≥3%.
All statistical analyses were performed using SPSS software (ver. 18.0 for Windows; SPSS, Inc., Chicago, IL., USA). Pearson's chi-squared test was used to assess the univariate association between clinical characteristics of the study population and sputum eosinophilia or AHR. Multiple logistic regression analysis was performed to obtain the adjusted odds ratios (OR) and 95% confidence intervals (CI) for the independent effect that sensitization to each allergen had on airway eosinophilic inflammation or AHR, adjusting for age, gender, current smoker, and the effect of relevant allergen exposure. Values of P<0.05 were considered to indicate statistical significance.
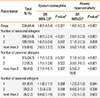
In total, 1,202 subjects with a mean age of 50.0±16.4 were included and Table 1 summarizes their baseline characteristics. Of the subjects, 520 (43.3%) were males and 534 (44.4%) had at least 1 positive skin test response to aeroallergens, considered as atopy. Sensitization to a perennial allergen was more frequent than sensitization to a seasonal allergen (40.1% versus 13.3%). The most commonly sensitized allergen was house dust mite, followed by cat and dog. AHR was demonstrated in 23.5% of the subjects and sputum eosinophilia in 38.8% (Table 1).
Table 2 shows the univariate analyses that explores the relationship between the clinical characteristics of the study population and sputum eosinophilia or AHR. Patients with AHR were younger, and current smokers were significantly more common. There was also a significant association between exposure to pets and sputum eosinophilia or AHR.
Multivariate analyses showed significant relationships between sputum eosinophilia and sensitization to at least 1 perennial allergen (OR, 1.9; 95% CI, 1.4-2.5), house dust mite (OR, 1.7; 95% CI, 1.3-2.3), dog (OR, 1.9; 95% CI, 1.1-3.3), and cat (OR, 2.1; 95% CI, 1.4 3.4; Table 3). Sputum eosinophilia showed increasing trends with higher grade responses in the skin prick test for D. farinae and cat (Fig. 1). Airway hyperresponsiveness was associated with sensitization to at least one perennial allergen (OR, 2.7; 95% CI, 2.0-3.7), house dust mite (OR, 2.2; 95% CI, 1.6 3.2), Alternaria (OR, 2.3; 95% CI, 1.2-4.7), and cat (OR, 2.7; 95% CI, 1.7-4.3; Table 4). The risk for AHR was greater in subjects with higher grade responses in the skin prick test for D. farinae and Alternaria (Fig. 2).
The relationship between sensitization to a seasonal allergen and sputum eosinophilia or AHR was not significant, even after adjusting for specific allergen exposure in the pollen season (Tables 3 and 4). The development of sputum eosinophilia and AHR examined in the relevant pollen season did not increase significantly compared with testing outside the pollen season (data not shown).
Sensitization to three or more allergens was related to increased sputum eosinophilia (OR, 2.0; 95% CI, 1.3-3.2) and AHR (OR, 3.6; 95% CI, 2.1-5.8; Table 5), although sensitization to 2 allergens showed a lower risk compared with sensitization to 1 allergen. Sputum eosinophilia and AHR showed an increasing trend with an increased number of positive test responses to perennial allergens (Table 5); however, there was no relationship with an increased number of sensitized seasonal allergens.
In the present study, the development of sputum eosinophilia or AHR was significantly associated with sensitization to perennial allergens such as house dust mite, Alternaria, dog, or cat. In addition, sputum eosinophilia and AHR showed increasing trends with sensitization to an increased number of perennial allergens.
Similar to the present study, many previous studies have shown relationships between specific allergen sensitization and bronchial hyperresponsiveness, with perennial indoor allergens such as house dust mite and pets being the most influential allergens.10,12,17-21 The present analysis showed an association between the sensitization to Alternaria and the development of AHR, as mentioned in some previous reports.22,23 The risk for AHR was greater in subjects with positive responses to perennial allergens compared to those who responded only to seasonal allergens;14 in addition, an increased wheal size in the skin test was significantly associated with asthma.24 The present results are consistent with previous results that showed that AHR increased as the number of sensitized allergens increased and the risk of eosinophilic airway inflammation and AHR increased with the increasing grades in skin prick test.14,24,25
In the present analysis, sensitization to seasonal allergens was not associated with airway eosinophilic inflammation or AHR. Previous studies have revealed that pollen allergy does not increase AHR.12,21,26 Some studies have a limitation that the subjects were tested outside of the pollen season where only the sensitization to perennial allergen was the risk factor for AHR and pollen sensitization did not contribute to the development of AHR or asthma.26 However, some studies have reported an association between sensitization to some seasonal allergens and AHR.11,18,27 Exposure to seasonal allergen during the pollen season was demonstrated to induce an inflammatory response in the airway and aggravate symptoms in pollen-sensitive asthmatics.28 Inconsistent results may be attributed to various factors such as differences in study population (age, presence of allergic rhinitis, or asthma), season of testing, and the multiple sensitization status of subjects. Studies conducted on children or younger adults often show an association between seasonal allergen sensitization and AHR; however, studies of older adults fail to reveal an association. Most studies have consistently demonstrated the relationship of perennial indoor allergen and asthma. Old adults with a mean age of 50 years were enrolled in our study. There was no increase in sputum eosinophils and no decrease in PC20 even in the subgroup analysis of the subjects tested during the relevant pollen season. The data suggest that seasonal pollen did not play a role in the development of AHR and eosinophilic airway inflammation unlike perennial allergens in the old adult population.
Another possible reason for the seemingly conflicting results related to seasonal allergens may be regional variations in allergens. Some evidence supports the effect of geographical climate variation on the prevalence and symptoms associated with respiratory allergic disease.29,30 Climate change can have an impact on the type, concentration, and allergenicity of aeroallergens as well as on the prevalence of allergic diseases.31 Geographic variation and regional types of allergens should be considered in the effect of allergic sensitization and the development of allergic disease.29
In respect to cockroach allergen, sensitization to cockroach was associated with the occurrence of asthma in other studies.9,32 However, the association was not found in the present study. Recently, cockroach infestation rates and sensitization rates are decreasing as the garbage disposal system has improved and insecticides are more commonly used in Korea.33 Reduced cockroach exposure (due to the improvement of hygienic conditions) might influence the lack of association between cockroach sensitization and AHR.
Different features of allergic diseases may be induced by the pattern of allergen sensitization. Compared to asthma patients, subjects with allergic rhinitis were more sensitized to allergens.13,25 Moreover, sensitization to outdoor allergens is associated with allergic rhinitis, rather than asthma;13,18 in particular, pollens were major sensitizing allergens in patients with allergic rhinitis.13,34 Factors that influenced the clinical significance of aeroallergens include concentration, particle size, and exposure duration of the aeroallergens.35,36 Airborne pollens with diameters larger than those of house dust mite particles may not reach the lower respiratory tract; however, conjunctival and upper respiratory tract mucosa can be exposed to large amounts of aeroallergens, even large allergens such as pollens. Additionally, indoor perennial allergens present in the environment year-round can induce chronic airway inflammation due to persistent challenges to the airway; subsequently, (compared to outdoor seasonal allergens) they can be the dominant allergens associated with asthma.2 Despite particle size, limited exposure period, and other special properties, pollens are still likely to trigger airway inflammation and AHR in special circumstances. Exposure to highly allergenic, submicron pollen particles or exposure to high amounts of pollen over an extended period can provoke allergic responses, even in the lower respiratory tract mucosa.37,38
The differences among the above studies may also be explained by an effect of protease activity from allergen sources on allergenicity.39-41 Proteases facilitate allergic sensitization by disrupting epithelial tight junctions and promoting the production of cytokines, chemokines, and adhesion molecules.37,42 Furthermore, proteases from mites and molds amplify IgE production, degranulate activated eosinophils, and can cause allergic inflammation in the respiratory system.39 Pollens also contain proteases and are thought to induce airway inflammation through protease-dependent and protease-independent mechanisms.43,44 However, the production of cytokines induced by pollens was lower than that induced by mite dust and a higher concentration of pollen was needed to produce a maximum concentration of cytokines.43 Thus, the protease activity of pollens in airway inflammation and AHR may be less potent than that of mite or mold allergens. A better understanding of the molecular characteristics of the allergenic properties of pollen would be helpful to elucidate the pathogenesis of allergic diseases.
There are several limitations to our study. First, the present study assessed only whether the subjects had been exposed to different allergens. Environmental exposure doses of each allergen and the exposure duration could not be investigated due to the retrospective nature of the study.45 Second, we included only patients who underwent all of the skin prick tests, a methacholine bronchial provocation test, and an induced sputum analysis. Patients who had no lower airway symptoms and had not undergone all of these tests were not included in the study. Thus, sputum eosinophilia and AHR were not evaluated in patients who had only rhinoconjuctivitis or asymptomatic allergen sensitization. Third, the risk of sputum eosinophilia and AHR was lower than sensitization to one allergen in subjects with sensitization to 2 allergens. This is thought to be because the subjects who were sensitized to 2 allergens had positive skin test responses with a lower A/H ratio. However, similar to previous reports, sensitization to 3 or more allergens showed a significant correlation with the development of sputum eosinophilia and AHR. Finally, the analysis of sputum eosinophilia was performed only once. A study by Simpson et al. indicated that a cut-off point of 3% eosinophils allows the presence of eosinophilia to be reliably determined from a single sputum sample.46 However, a recent study suggested that sputum inflammatory types are not stable over time.47 Single induced sputum analysis may have a limitation to determine the presence of sputum eosinophilia.
In conclusion, the development of airway eosinophilic inflammation and AHR in a Korean adult population was associated with sensitization to perennial allergens, as opposed to outdoor seasonal allergens. Understanding the pattern of allergen sensitization would be helpful to determine which clinical features of allergic disease would be expected to develop.
Figures and Tables
Fig. 1
Adjusted odds ratios (95% CI) of airway eosinophilic inflammation in relation to grades of skin prick test. *Statistical comparison was performed with group with negative skin test response to each grade of allergen; the odds ratio and P value were adjusted according to age, gender, and smoking status.

Fig. 2
Adjusted odds ratios (95% CI) of airway hyperresponsiveness in relation to grades of skin prick test. *Statistical comparison was performed with group with negative skin test response to each grade of allergen; the odds ratio and P value were adjusted according to age, gender, and smoking status.

Table 1
Clinical characteristics of the study population (N=1,202)

Table 2
Relationship between baseline characteristics and sputum eosinophilia or airway hyperresponsiveness

Table 3
Relationship between aeroallergen sensitization and sputum eosinophilia

Table 4
Relationship between aeroallergen sensitization and airway hypersensitivity

Table 5
Sputum eosinophilia and airway hyperresponsiveness according to atopy status
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
1. Levetin E, Van de Water P. Environmental contributions to allergic disease. Curr Allergy Asthma Rep. 2001; 1:506–514.
2. Platts-Mills TA, Blumenthal K, Perzanowski M, Woodfolk JA. Determinants of clinical allergic disease. The relevance of indoor allergens to the increase in asthma. Am J Respir Crit Care Med. 2000; 162:S128–S133.
3. Dreborg S. Impact of allergen exposure on bronchial hyperresponsiveness. Pediatr Allergy Immunol. 1996; 7:14–17.
4. Brightling CE. Clinical applications of induced sputum. Chest. 2006; 129:1344–1348.
5. Chung KF. Role of inflammation in the hyperreactivity of the airways in asthma. Thorax. 1986; 41:657–662.
6. Alvarez MJ, Olaguibel JM, García BE, Rodríquez A, Tabar AI, Urbiola E. Airway inflammation in asthma and perennial allergic rhinitis. Relationship with nonspecific bronchial responsiveness and maximal airway narrowing. Allergy. 2000; 55:355–362.
7. Corren J. Allergic rhinitis and asthma: how important is the link? J Allergy Clin Immunol. 1997; 99:S781–S786.
8. Jaakkola MS, Ieromnimon A, Jaakkola JJ. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol. 2006; 117:642–648.
9. Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001; 107:419–428.
10. Chinn S, Heinrich J, Antó JM, Janson C, Norbäck D, Olivieri M, Svanes C, Sunyer J, Verlato G, Wjst M, Zock JP, Burney PG, Jarvis DL. Bronchial responsiveness in atopic adults increases with exposure to cat allergen. Am J Respir Crit Care Med. 2007; 176:20–26.
11. Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989; 19:419–424.
12. Omenaas E, Bakke P, Eide GE, Elsayed S, Gulsvik A. Serum house dust mite antibodies: predictor of increased bronchial responsiveness in adults of a community. Eur Respir J. 1996; 9:919–925.
13. Boulet LP, Turcotte H, Laprise C, Lavertu C, Bédard PM, Lavoie A, Hébert J. Comparative degree and type of sensitization to common indoor and outdoor allergens in subjects with allergic rhinitis and/or asthma. Clin Exp Allergy. 1997; 27:52–59.
14. Craig TJ, King TS, Lemanske RF Jr, Wechsler ME, Icitovic N, Zimmerman RR Jr, Wasserman S. National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Aeroallergen sensitization correlates with PC(20) and exhaled nitric oxide in subjects with mild-to-moderate asthma. J Allergy Clin Immunol. 2008; 121:671–677.
15. Oh JW, Lee HB, Kang IJ, Kim SW, Park KS, Kook MH, Kim BS, Baek HS, Kim JH, Kim JK, Lee DJ, Kim KR, Choi YJ. The revised edition of Korean calendar for allergenic pollens. Allergy Asthma Immunol Res. 2012; 4:5–11.
16. Sohn SW, Lee HS, Park HW, Chang YS, Kim YK, Cho SH, Kim YY, Min KU. Evaluation of cytokine mRNA in induced sputum from patients with allergic rhinitis: relationship to airway hyperresponsiveness. Allergy. 2008; 63:268–273.
17. Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003; 112:362–368.
18. Tepas EC, Litonjua AA, Celedón JC, Sredl D, Gold DR. Sensitization to aeroallergens and airway hyperresponsiveness at 7 years of age. Chest. 2006; 129:1500–1508.
19. Verdiani P, Di Carlo S, Baronti A. Different prevalence and degree of nonspecific bronchial hyperreactivity between seasonal and perennial rhinitis. J Allergy Clin Immunol. 1990; 86:576–582.
20. Mete N, Sin A, Gulbahar O, Erdinc M, Sebik F, Kokuludag A. The determinants of bronchial hyperresponsiveness in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2004; 93:193–199.
21. Kerkhof M, Postma DS, Schouten JP, de Monchy JG. Allergic sensitization to indoor and outdoor allergens and relevance to bronchial hyperresponsiveness in younger and older subjects. Allergy. 2003; 58:1261–1267.
22. Peat JK, Tovey E, Mellis CM, Leeder SR, Woolcock AJ. Importance of house dust mite and Alternaria allergens in childhood asthma: an epidemiological study in two climatic regions of Australia. Clin Exp Allergy. 1993; 23:812–820.
23. Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leüppi JD, Xuan W, Downie SR, Tobias A, Peat JK. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001; 164:455–459.
24. Nelson HS. The importance of allergens in the development of asthma and the persistence of symptoms. J Allergy Clin Immunol. 2000; 105:S628–S632.
25. Pallasaho P, Rönmark E, Haahtela T, Sovijärvi AR, Lundbäck B. Degree and clinical relevance of sensitization to common allergens among adults: a population study in Helsinki, Finland. Clin Exp Allergy. 2006; 36:503–509.
26. Patelis A, Gunnbjörnsdottir M, Malinovschi A, Matsson P, Onell A, Högman M, Alving K, Janson C. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012; 130:397–402.e2.
27. Chinn S, Burney P, Sunyer J, Jarvis D, Luczynska C. Sensitization to individual allergens and bronchial responsiveness in the ECRHS. European Community Respiratory Health Survey. Eur Respir J. 1999; 14:876–884.
28. Djukanović R, Feather I, Gratziou C, Walls A, Peroni D, Bradding P, Judd M, Howarth PH, Holgate ST. Effect of natural allergen exposure during the grass pollen season on airways inflammatory cells and asthma symptoms. Thorax. 1996; 51:575–581.
29. Zanolin ME, Pattaro C, Corsico A, Bugiani M, Carrozzi L, Casali L, Dallari R, Ferrari M, Marinoni A, Migliore E, Olivieri M, Pirina P, Verlato G, Villani S, Marco R. ISAYA Study Group. The role of climate on the geographic variability of asthma, allergic rhinitis and respiratory symptoms: results from the Italian study of asthma in young adults. Allergy. 2004; 59:306–314.
30. Garcia-Marcos L, Garcia-Hernández G, Morales Suarez-Varela M, Batlles Garrido J, Castro-Rodriguez JA. Asthma attributable to atopy: does it depend on the allergen supply. Pediatr Allergy Immunol. 2007; 18:181–187.
31. Beggs PJ. Impacts of climate change on aeroallergens: past and future. Clin Exp Allergy. 2004; 34:1507–1513.
32. Sohn MH, Kim KE. The cockroach and allergic diseases. Allergy Asthma Immunol Res. 2012; 4:264–269.
33. Yong TS, Jeong KY. Household arthropod allergens in Korea. Korean J Parasitol. 2009; 47:Suppl. S143–S153.
34. Sakashita M, Hirota T, Harada M, Nakamichi R, Tsunoda T, Osawa Y, Kojima A, Okamoto M, Suzuki D, Kubo S, Imoto Y, Nakamura Y, Tamari M, Fujieda S. Prevalence of allergic rhinitis and sensitization to common aeroallergens in a Japanese population. Int Arch Allergy Immunol. 2010; 151:255–261.
35. Platts-Mills TA, Heymann PW, Longbottom JL, Wilkins SR. Airborne allergens associated with asthma: particle sizes carrying dust mite and rat allergens measured with a cascade impactor. J Allergy Clin Immunol. 1986; 77:850–857.
36. Schappi GF, Taylor PE, Pain MC, Cameron PA, Dent AW, Staff IA, Suphioglu C. Concentrations of major grass group 5 allergens in pollen grains and atmospheric particles: implications for hay fever and allergic asthma sufferers sensitized to grass pollen allergens. Clin Exp Allergy. 1999; 29:633–641.
37. Kauffman HF. Interaction of environmental allergens with airway epithelium as a key component of asthma. Curr Allergy Asthma Rep. 2003; 3:101–108.
38. D'Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007; 62:976–990.
39. Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004; 114:997–1008.
40. Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998; 19:313–316.
41. Gunawan H, Takai T, Ikeda S, Okumura K, Ogawa H. Protease activity of allergenic pollen of cedar, cypress, juniper, birch and ragweed. Allergol Int. 2008; 57:83–91.
42. Holgate ST. Airway inflammation and remodeling in asthma: current concepts. Mol Biotechnol. 2002; 22:179–189.
43. Tomee JF, van Weissenbruch R, de Monchy JG, Kauffman HF. Interactions between inhalant allergen extracts and airway epithelial cells: effect on cytokine production and cell detachment. J Allergy Clin Immunol. 1998; 102:75–85.
44. Hassim Z, Maronese SE, Kumar RK. Injury to murine airway epithelial cells by pollen enzymes. Thorax. 1998; 53:368–371.
45. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997; 336:1356–1363.
46. Simpson JL, McElduff P, Gibson PG. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration. 2010; 79:147–151.
47. Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012; 67:675–681.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download