Abstract
Purpose
The role of systemic inflammation on asthma-COPD overlap syndrome is unknown. This study aimed to examine systemic inflammation in asthma-COPD overlap syndrome, and to identify associations between clinical characteristics and inflammatory mediators in asthma-COPD overlap syndrome.
Methods
In 108 adults older than 55 years comprising healthy controls (n=29), asthma (n=16), COPD (n=21) and asthma-COPD overlap syndrome (n=42), serum high sensitivity C-reactive protein and Interleukin 6 (IL-6) were assayed. Spirometry, induced sputum, quality of life, comorbidities and medications were assessed, and their associations with asthma-COPD overlap syndrome were analyzed using logistic regression. Associations between systemic inflammatory mediators and clinical characteristics were tested in multivariate linear regression models.
Results
Patients with asthma-COPD overlap syndrome had significantly elevated IL-6 levels compared with healthy controls and asthmatics. Age, comorbidity index and IL-6 level were independently associated with asthma-COPD overlap syndrome. FEV1% predicted was inversely associated with IL-6 level, and cardiovascular disease was associated with an increased IL-6 level. Systemic markers were not associated with airway inflammation.
Conclusions
Systemic inflammation is commonly present in asthma-COPD overlap syndrome, and asthma-COPD overlap syndrome resembled COPD in terms of systemic inflammation. IL-6 is a pivotal inflammatory mediator that may be involved in airflow obstruction and cardiovascular disease and may be an independent treatment target.
With ageing, there is an increasing overlap of asthma and chronic obstructive pulmonary disease (COPD), such that many older patients with obstructive airway disease (OAD) have features of both asthma and COPD, termed asthma-COPD overlap syndrome.1 Hardin et al. reports that outcomes including health-related quality of life (HRQoL) impairment are worse for people with asthma-COPD overlap syndrome than those with COPD alone, and that these patients were more likely to experience frequent and severe exacerbations compared with COPD patients.2
Systemic inflammation is a feature of chronic inflammatory airway disease. While it is typically associated with COPD and ageing,3 systemic inflammation has recently been linked to some forms of asthma.4 In COPD, systemic inflammation with chronic low-grade elevation of circulating pro-inflammatory mediators such as C-reactive protein (CRP) and interleukin 6 (IL-6) is associated with accelerated disease progression, characterized by acute exacerbations,5 COPD-related hospitalization,6 and rapid decline of force expiratory volume in 1 second (FEV1).6 These same markers of systemic inflammation are also associated with ageing and comorbid diseases, such as cardiovascular disease (CVD),7 obesity,8 diabetes,8 and are believed to actively participate in the pathogenesis of these conditions.
Given that the prevalence of asthma-COPD overlap syndrome increases with age, it is unclear whether systemic inflammation is also a feature of this condition, and if so, which markers are involved in and what are the clinical correlates of these markers. The impact of the confounding effects of age, CVD and obesity on systemic inflammation in asthma-COPD overlap syndrome is also unknown. Systemic inflammation may also be a contributing factor to the increased morbidity (decreased quality of life, increased admission and greater decline in lung function) associated with the asthma-COPD overlap syndrome reported by Hardin et al.2
In this study we have therefore examined levels of systemic inflammatory mediators in asthma, COPD and asthma-COPD overlap syndrome, and identified potential clinical characteristics that were associated with levels of these markers. We have also examined clinical correlates and inflammatory markers that were associated with the asthma-COPD overlap syndrome, and tested the hypothesis that systemic inflammation is present in asthma-COPD overlap syndrome, and independently associated with clinical characteristics of obstructive airway disease.
A cross-sectional study was conducted, involving 108 older people (>55 years of age) who underwent a multidimensional assessment of clinical and functional status and comorbidity.9 Baseline clinical characteristic data from this study are published elsewhere.10 Participants gave written informed consent. The Hunter New England Area Health Service and University of Newcastle Research Human Ethics Committees approved this study.
We recruited a total of 39 participants (age >55 years) without airways disease (healthy controls) by community advertisement. One hundred and eleven participants with obstructive airways disease were consecutively recruited according to physician diagnosis and spirometry from the Respiratory and Sleep Medicine Ambulatory Care Clinics at the John Hunter Hospital, Newcastle NSW, Australia. Patients that were excluded from the analysis if they were current smokers or had ceased within the previous 12 months, had other respiratory disease, and were participants who failed to have blood samples collected. Healthy controls were excluded if their spirometry was outside normal limits. After exclusions were applied, 79 patients with OAD and 29 healthy controls were included in this analysis (Fig. 1).
Diagnostic classification was performed using standardized objective criteria (Table 1).1 Participants in the healthy group had no diagnosed airway disease, confirmed by a normal lung function with post bronchodilator FEV1/FVC ≥70% and post-bronchodilator FEV1 >80% of predicted. Asthma was defined as episodic respiratory symptoms and reversible airflow obstruction with a post bronchodilator FEV1/FVC ≥70% and post-bronchodilator FEV1 >80% of predicted with airway hyper-responsiveness (AHR) or bronchodilator hyper-responsiveness (BHR). Participants with COPD had incompletely reversible airflow obstruction with a post bronchodilator FEV1/FVC <70% and post-bronchodilator FEV1 <80% of predicted and no AHR or bronchodilator responsiveness (BDR). Participants with the asthma-COPD overlap syndrome had respiratory symptoms, increased airflow variability (asthma, AHR or BDR) as well as incompletely reversible airflow obstruction (COPD, post bronchodilator FEV1/FVC <70% and post-bronchodilator FEV1 <80% of predicted).
Participants attended three visits within one month to complete their assessments. Pre and post bronchodilator spirometry, symptoms, smoking status, medical history, and medication use were assessed at visit one and a sputum induction was undertaken. At visit 2, venepuncture with blood collection and a skin allergy test11 was performed, and sputum induction was repeated if sputum sample failed to be obtained in the first visit. On the final visit a hypertonic saline (4.5%) challenge was performed in those whose visit 1 FEV1 was >1.3 L as previously described.12
Questionnaires were completed which assessed respiratory symptoms such as cough, breathlessness, wheeze and mucus production. A smoking history was taken and smoking pack-years determined. Health status was measured using St George's Respiratory Questionnaire (SGRQ).13 The Charlson Co-morbidity Index (CCI) was calculated using information collected in the medical history.14
Participants withheld bronchodilators for their duration of action before testing. Three reproducible measurements of FEV1 and FVC were obtained (KoKo PD Instrumentation, Louisville, CO, USA) before and after inhalation of 400 mcg salbutamol via a metered dose inhaler with valved holding chamber (Volumatic, Allen and Hanbury's, Melbourne Victoria, Australia). Predicted values were calculated according to Knudson et al.15
Standard venepuncture and infection prevention standards were used during sample collection. Peripheral venous blood was collected into Vacutainer® tubes (BD Worldwide, North Ryde, NSW, Australia). High sensitivity CRP (MP Biomedicals, Australasia, Seven Hills, NSW, Australia) and IL-6 (R&D Systems, Minneapolis, MN, USA) were determined in duplicate using enzyme-linked immunosorbent assay according to the manufacturers' instructions. Elevated CRP and IL-6 levels were defined as CRP >4.12 mg/L and IL-6 >1.55 pg/mL based on prior data.4
Data were analyzed using Stata 11 (Stata Corporation, College Station, Texas, USA). Results are reported as median and interquartile range (IQR) unless otherwise indicated. Analysis was performed using One-way ANOVA or Kruskal-Wallis test for more than 2 groups, as appropriate. Post-hoc analysis with Bonferroni adjustment was conducted for One-way ANOVA test, or using adjusted P<0.008 for Kruskal-Wallis test on statistically significant groups. The two-sample Wilcoxon Rank Sum test was used to test non-parametric data between 2 independent groups, and the Fishers' exact test for categorical data. Independent predictors for asthma-COPD overlap syndrome were identified using univariate logistic regression followed by a multivariate logistic regression analysis. Variables that were tested in univariate logistic regression with P<0.1 were selected in multivariate logistic regression model. Demographic variables including age and sex were forced into multivariate regression analysis. Associations between systemic inflammatory mediators and clinical characteristics were determined using single and multiple linear regression. Levels of CRP and IL-6 were natural log transformed and reciprocal square roots transformed to normality, respectively. Results are reported as significant when P<0.05.
In 79 subjects with obstructive airways disease, there were 16 (20.3%) with asthma, 21 (26.6%) with COPD and 42 (53.2%) with asthma-COPD overlap syndrome (Table 2). The four groups had similar BMI (P=0.316), atopic status (P=0.108), inhaled corticosteroid use (P=0.516), history of cardiac dysfunction (P=0.573) and HMGCoA reductase inhibitor (statin) use (P=0.169). Most asthmatics in this study were never smokers, whereas 76.2% of participants in the COPD group (P=0.006) and 71.4% in the asthma-COPD overlap syndrome group (P=0.005) were ex-smokers.
Subjects with COPD and asthma-COPD overlap syndrome had significantly impaired lung function compared with healthy controls and the asthmatics, for both mean FEV1% predicted and FEV1/FVC ratio ranging from 50% to 55% (P<0.001). FEV1% predicted alone in asthma group was lower than that in healthy controls (P=0.004). The airway disease groups had higher CCI when compared with healthy controls (P<0.001), but there was no difference within the disease groups (P=0.130) (Table 2).
Sputum eosinophil and neutrophil counts were different among the 4 groups as Table 2 shows, however no significant difference was found between any 2 groups comparison in the post-hoc analysis with the exception of a higher sputum eosinophil number in the asthma-COPD overlap syndrome group compared to healthy controls (P=0.002).
There was evidence of systemic inflammation in asthma-COPD overlap syndrome groups (Table 3). CRP levels were different among the 4 groups (P=0.020). Median CRP level was higher in patients with COPD and asthma-COPD overlap syndrome but the difference was not significant compared with healthy controls and asthma groups. The proportion of subjects with an elevated CRP level was not different among the 4 groups (P=0.064).
IL-6 levels were highest in the asthma-COPD overlap syndrome group (Fig. 2), and were significantly elevated compared to healthy controls (P=0.006) and the asthma group (P=0.0086). Significantly more people with asthma-COPD overlap syndrome had increased IL-6 levels (81.0%) compared to asthma (37.5%, P=0.001). No difference was found in IL-6 level (P=0.688) or proportion of patients with elevated IL-6 (P=0.209) between COPD and asthma-COPD overlap syndrome groups (Table 3).
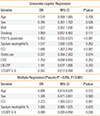
We performed multivariate logistic regression analysis to determine which clinical feature could be independently associated with asthma-COPD overlap syndrome (Table 4). In the univariate logistic regression analysis, FEV1% predicted, sputum neutrophil counts, CCI and IL-6 level significantly predicted the risk of having asthma-COPD overlap syndrome (P<0.1). FEV1% predicted was removed from the multivariate regression model due to the physiological classification of asthma-COPD overlap syndrome. In a multivariate regression model adjusting for confounders, the associations of age (adjusted OR=0.894, P=0.012) and 1/SQRT IL-6 level (adjusted OR=0.089, P=0.036) with a decreased likelihood of asthma-COPD overlap syndrome were identified. Co-morbidity measured by CCI (adjusted OR=2.223, P=0.001) was associated with an increased likelihood of having asthma-COPD overlap syndrome in the model independent of other variables.
In univariate regression, serum CRP level (LN CRP) was associated with BMI (Coef.=0.059, 95% CI [0.027, 0.090], P<0.001), FEV1% predicted (Coef.=-0.012, 95% CI [-0.020, -0.005], P=0.002), sputum neutrophil counts (Coef.=0.014, 95% CI [0.005, 0.023], P=0.003) and CCI (Coef.=0.173, 95% CI [0.057, 0.289], P=0.004), however, when adjusted with age, sex, smoking history, statin use and CVD in a multivariate regression model (R2=0.245, P=0.004), BMI was the only clinical characteristic that was associated with CRP level (Coef.=0.052, 95% CI [0.016, 0.089], P=0.005).
IL-6 level was negatively associated with FEV1% predicted (calculated as 1/SQRT IL-6, Coef.=0.004, 95% CI [0.002, 0.005], P<0.001), and was positively associated with age (Coef.=-0.011, 95% CI [-0.017, -0.004], P=0.002), sputum neutrophil percentage (Coef.=-0.003, 95% CI [-0.005, -0.001], P=0.005) and concurrence of CVD (Coef.=-0.227, 95% CI [-0.349, -0.104], P<0.001) in univariate regression. Correlation between IL-6 level and FEV1% predicted is shown in Fig. 3A. Subjects with CVD had higher IL-6 levels (4.1, 2.7-5.0) than those without CVD history (1.9, 1.2-3.4) (P<0.001, Fig. 3B). After controlling for sex, BMI, smoking history, CCI and statin use in a linear regression model (R2=0.436, P<0.001), age (Coef.=-0.012, 95% CI [-0.019, -0.004], P=0.002), FEV1% predicted (Coef.=0.004, 95% CI [0.001, 0.006], P=0.004) and CVD (Coef.=-0.179, 95% CI [-0.308, -0.050], P=0.007) were still significant predictors for IL-6 level, but not sputum neutrophil counts (Coef.=-0.0003, 95% CI [-0.002, 0.002], P=0.745).
This study assessed systemic inflammation in patients with obstructive airway diseases including asthma, COPD and asthma-COPD overlap syndrome and healthy controls. Our data shows that systemic inflammation is frequently present in asthma-COPD overlap syndrome, with a similar prevalence to COPD, where it is present in between 47.6% and 81.0% of patients. In asthma-COPD overlap syndrome, elevated IL-6 was common, and after adjusting for confounding factors, systemic levels of IL-6 was strongly associated with FEV1% predicted and cardiovascular disease. Multivariate logistic regression modeling suggests that age, CCI and IL-6 level were associated with asthma-COPD overlap syndrome.
Asthma-COPD overlap syndrome is common in patients with obstructive airway disease, particularly in older people, and its prevalence continues to increase with age.16,17 In this study, there were 53.2% of subjects with an overlapping pattern of airflow obstruction. These data support the high prevalence of asthma-COPD overlap syndrome reported in the study by Marsh et al., in which 55% of COPD patients present an asthma predominant phenotype.18 In the majority of previous reports of systemic inflammation in chronic airway disease, patients with asthma-COPD overlap syndrome were either not properly defined,19,20,21 or were specifically excluded, this may result in mixed study population of patients with (A) fixed airflow obstruction and, (B) both airflow reversibility and fixed obstruction, which may represent different disease pathogenesis. Furthermore, smoking history was used as an exclusion criterion and as a result a great number of patients were excluded from the studies.19,20,21,22 It is well known that studies, in particular randomised controlled trials (RCTs), in asthma and COPD are based on highly selected subgroups of people, however this does not reflect the spectrum of patient seen in practice23,24 leading to evidence gaps. Real world studies such as ours that have examined a wide spectrum of obstructive airway disease patients and specially include and analyse the asthma-COPD overlap phenotype are needed in order to inform practice. Therefore, this study provides novel data in relation to this patient population.
We have addressed the prevalence and role of systemic inflammation in asthma-COPD overlap syndrome, an observation that has received little attention elsewhere. CRP is an acute phase protein present in various pathological conditions,25 and is frequently used as a surrogate marker for inflammation. IL-6 is a pro-inflammatory cytokine and is the primary hepatocyte stimulating factor for acute phase proteins.26 We choose to determine the two inflammatory markers because both have been commonly studied in COPD and in some asthma studies.4,27 Our data shows that patients with asthma-COPD overlap syndrome had significantly higher IL-6 levels, and there were a greater proportion of patients in this group with elevated IL-6 levels compared to the asthma group, however, levels of systemic inflammation mediators did not differ between COPD and asthma-COPD overlap syndrome. Thus, the common presence of systemic inflammation seen in asthma-COPD overlap syndrome, together with the similar levels of systemic inflammatory mediators with the COPD group, suggest that this group resembles COPD in terms of systemic inflammation.
Elevated levels of systemic inflammatory mediators are attributed to multiple factors, such as aging,3 cigarette smoking,28 obesity,8 cardiovascular diseases,7 other co-morbidities8 and statin use.29 We used multivariate linear regression analysis in order to control for the effects of these confounders on levels of systemic inflammatory mediators. After adjustment, BMI was independently associated with CRP level. CRP levels were associated with FEV1% predicted in single linear regression, however when adjusted for other confounders, the association was no longer significant, suggesting that CRP may not be a major marker of airflow obstruction. This differs from some other studies,30 perhaps because of different populations studied, and limited confounders adjusted for in those studies.4 The present study examined a wide range of potential confounders for the frequently reported inflammatory mediators CRP and IL-6, which led to different associations being identified; we therefore argue that this approach is rational and pivotal in the assessment of systemic inflammation.
Our data show that after controlling for other confounders, FEV1% predicted was significantly and inversely correlated with IL-6 level. Walter et al.31 found that serum IL-6 is inversely related to FEV1 independent of age and smoking, and high levels of IL-6 were associated with increased risk of developing COPD.32 The population-based Burden of Obstructive Lung Disease study30 suggests that IL-6 was independently related to lower FEV1 and FVC value, and was associated with a faster FEV1% predicted decline in COPD. Interestingly, an earlier study has shown that Oncostatin M, a member of IL-6 family of cytokines,33 was associated with incompletely reversible airflow obstruction in asthma via modulating airway inflammation.34 In our study the elevation of IL-6 level was seen in the asthma-COPD overlap syndrome group but not in the 'pure asthma' group compared to healthy controls, it is possible that the elevation of IL-6 level is associated with an fixed airflow obstruction in asthma, i.e. an overlap pattern, rather than as an universal increase in all the asthmatics, and this requires further study.
We did not find a difference in IL-6 levels between those with asthma and healthy controls, these data support the results of an early study35 but conflict with some other studies.4,36,37 The reason of this is unclear but may due to small number of the patients in the asthma group in our study. Moreover, the patient population and also the healthy controls in this study were older than those recruited in previous studies.4,36,37 Finally, we used strict physiological criteria to define each group and the patients in the asthma group had AHR or BDR without a coexisting of airflow obstruction. In previous studies,4,36,37 patients with fixed airflow obstruction were not excluded from the asthma population. This results in a mixed study population with asthma and overlap syndrome in their 'asthma group', and may contribute to the elevation of IL-6 level compared to healthy controls seen in those studies.
In the logistic regression model of asthma-COPD overlap syndrome, age, CCI and IL-6 level were significantly associated with this specific phenotype. Relatively early onset of asthma symptoms and long-term airway inflammation and remodeling may lead to an irreversible airflow obstruction in asthma-COPD overlap syndrome with younger age being identified in this study. After controlling for other factors, an elevation of IL-6 was also significantly associated with the overlap syndrome, indicating a pivotal role of IL-6 in asthma-COPD overlap syndrome. Additionally, higher levels of IL-6 in patients with CVD compared to those without CVD, together with the inverse correlation between the IL-6 level and FEV1% predicted, may indicate that IL-6 is a potentially important systemic inflammatory mediator that is involved in the lung function impairment and comorbidities in obstructive airway disease.
There are some limitations of this study. We observed a relatively small number of patients for the asthma group. Asthma is a potential risk factor for developing COPD, and persistent airway inflammation and the subsequent airway remodeling in asthma may result in an incompletely reversible airflow obstruction in older asthmatics.38 Since this may represent the natural history of asthma physiologically, identifying fewer patients as 'pure asthma' in this older population is not unexpected. Additionally, the consistence of the proportion of patients that had asthma-COPD overlap syndrome with previously published studies18,39 indicates the proportion of the patients in each group is not likely due to an intentional selection. The application of the strict physiological criteria to define the disease subgroups may also contributed to the small simple size, in the asthma group particularly. Future studies with a larger sample size are required to confirm the research findings. We measured two of the typical mediators to assess systemic inflammation, in future studies measurement a panel of biomarkers is suggested.40 Moreover, to investigate the predictors for developing asthma-COPD overlap syndrome and prognostic role of systemic inflammation in OAD, a longitudinal study will be valuable.
At present there is no agreed definition for the diagnosis of asthma-COPD overlap syndrome. Other definitions have been proposed based on the presence of specific airway inflammatory pattern,41 but these inflammatory phenotypes are not specific for asthma and COPD such as airway eosinophilia was present only in 48% of asthmatics, while 34% of patients with COPD had an eosinophilic inflammation.42 We used the physiological lung function criteria with the presence where possible of AHR.1 Most patients in asthma-COPD overlap syndrome group (92.7%) showed bronchial reactivity to hypertonic saline, which is an indirect bronchial provocation agent with greater specificity for asthma,43 and is less susceptible to the effects of baseline airflow obstruction compared to direct acting agents (e.g. methacholine).44 It also had the advantage of permitting collection of induced sputum. In patients whose lung function was too low to undergo a hypertonic saline challenge we used the presence of BDR in the definition of asthma-COPD overlap syndrome. In the absence of better diagnostic criteria, we urge that this is justified as the overlap group had clinical symptoms and physiological changes of asthma and COPD. Additionally, the concordance with the proportion of patients that had asthma-COPD overlap syndrome with other studies18,39 may also indicate the rationality of this definition. Validation of the definition is required to promote a consensus in the respiratory community.
In conclusion, our data identified a high prevalence of systemic inflammation in older people with the asthma-COPD overlap syndrome. Patients with asthma-COPD overlap syndrome resembled COPD in terms of systemic inflammation. Age, CCI and serum IL-6 level were independently associated with asthma-COPD overlap syndrome. Elevated IL-6 was significantly elevated in asthma-COPD overlap syndrome compared with healthy controls and asthma, and was independently associated with the degree of lung function impairment and cardiovascular disease, therefore might be an important inflammatory biomarker that is associated with airflow obstruction and the systemic effects observed. Further research on systemic inflammation in this population would improve our understanding and provide potential treatment targets for older people with obstructive airway disease.
Figures and Tables
Fig. 3
(A) Correlation between FEV1% predicted with IL-6, (B) Levels of IL-6 in patients with CVD vs non-CVD.

Table 1
Physiological characteristics and definition of obstructive airway syndrome

| Healthy Control | Asthma | COPD | Asthma-COPD overlap syndrome | |
|---|---|---|---|---|
| Symptoms* | - | + | + | + |
| Post bronchodilator FEV1/FVC% | ≥70 | ≥70 | <70 | <70 |
| Post bronchodilator FEV1% predicted | >80 | >80 | <80 | <80 |
| AHR or BDR | - | + | - | + |
Table is reproduced from reference (1), by permission of the journal Thorax.
*At least one of the following respiratory symptoms: breathlessness, wheeze, cough, sputum production. Airway Hyperresponsiveness (AHR), FEV1 fall≥15% from baseline after inhalation of 4.5% hypertonic saline. Bronchodilator responsiveness (BDR), post-bronchodilator FEV1 increased ≥200 mL and 12% compared with pre-bronchodilator FEV1.45
Table 2
Participant characteristics by diagnostic group
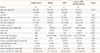
Table 3
Levels of systemic inflammatory mediators in different groups

Table 4
Logistic regression to identify risk factors for asthma-COPD overlap syndrome
ACKNOWLEDGMENTS
We acknowledge the technical support from Kelly Steel, Calida Garside, Erin Harvey, Kevin Oreo and Michelle Gleeson. This work was financially supported by the National Health and Medical Research Council, and the National Health and Medical Research Council Centre for Respiratory and Sleep Medicine, the Co-operative Research Centre (CRC) for Asthma and Airways, Australia. The financial sponsors played no role in the design, execution, analysis and interpretation of data or writing of the study.
References
1. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009; 64:728–735.
2. Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, Crapo JD, Hersh CP. COPDGene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011; 12:127.
3. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005; 579:2035–2039.
4. Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012; 142:86–93.
5. Dickens JA, Miller BE, Edwards LD, Silverman EK, Lomas DA, Tal-Singer R. Evaluation of COPD Longitudinally to Identify Surrogate Endpoints (ECLIPSE) study investigators. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res. 2011; 12:146.
6. Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007; 175:250–255.
7. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004; 350:1387–1397.
8. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17:4–12.
9. Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010; 376:803–813.
10. McDonald VM, Simpson JL, Higgins I, Gibson PG. Multidimensional assessment of older people with asthma and COPD: clinical management and health status. Age Ageing. 2011; 40:42–49.
11. Dreborg S. Bronchial hyper-reactivity and skin sensitivity. Clin Exp Allergy. 1991; 21:529.
12. Gibson PG, Wlodarczyk JW, Hensley MJ, Gleeson M, Henry RL, Cripps AW, Clancy RL. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med. 1998; 158:36–41.
13. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992; 145:1321–1327.
14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383.
15. Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976; 113:587–600.
16. Zeki AA, Schivo M, Chan A, Albertson TE, Louie S. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo). 2011; 2011:861926.
17. McDonald VM, Higgins I, Gibson PG. Managing older patients with coexistent asthma and chronic obstructive pulmonary disease: diagnostic and therapeutic challenges. Drugs Aging. 2013; 30:1–17.
18. Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, Hansell AL, Nowitz MR, McNaughton AA, Soriano JB, Beasley RW. Proportional classifications of COPD phenotypes. Thorax. 2008; 63:761–767.
19. Higashimoto Y, Yamagata Y, Taya S, Iwata T, Okada M, Ishiguchi T, Sato H, Itoh H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: similarities and differences. Respirology. 2008; 13:128–133.
20. Eagan TM, Ueland T, Wagner PD, Hardie JA, Mollnes TE, Damås JK, Aukrust P, Bakke PS. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J. 2010; 35:540–548.
21. Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010; 11:77.
22. Wu TL, Chang PY, Tsao KC, Sun CF, Wu LL, Wu JT. A panel of multiple markers associated with chronic systemic inflammation and the risk of atherogenesis is detectable in asthma and chronic obstructive pulmonary disease. J Clin Lab Anal. 2007; 21:367–371.
23. Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, Aldington S, Beasley R. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007; 62:219–223.
24. Travers J, Marsh S, Caldwell B, Williams M, Aldington S, Weatherall M, Shirtcliffe P, Beasley R. External validity of randomized controlled trials in COPD. Respir Med. 2007; 101:1313–1320.
25. Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006; 119:166.e17–166.e28.
26. Heinrich PC, Horn F, Graeve L, Dittrich E, Kerr I, Müller-Newen G, Grötzinger J, Wollmer A. Interleukin-6 and related cytokines: effect on the acute phase reaction. Z Ernahrungswiss. 1998; 37:Suppl 1. 43–49.
27. Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, Jinnai M, Muro S, Hirai T, Ito Y, Nakamura T, Mio T, Chin K, Mishima M. High sensitivity C-reactive protein in asthma. Eur Respir J. 2006; 27:908–912.
28. Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, Celli BR. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006; 61:23–28.
29. Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can J Cardiol. 2010; 26:Suppl A. 41A–44A.
30. Thorleifsson SJ, Margretardottir OB, Gudmundsson G, Olafsson I, Benediktsdottir B, Janson C, Buist AS, Gislason T. Chronic airflow obstruction and markers of systemic inflammation: results from the BOLD study in Iceland. Respir Med. 2009; 103:1548–1553.
31. Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF Jr, Lipinska I, O'Connor GT, Benjamin EJ. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008; 133:19–25.
32. Yanbaeva DG, Dentener MA, Spruit MA, Houwing-Duistermaat JJ, Kotz D, Passos VL, Wouters EF. IL6 and CRP haplotypes are associated with COPD risk and systemic inflammation: a case-control study. BMC Med Genet. 2009; 10:23.
33. O'Hara KA, Kedda MA, Thompson PJ, Knight DA. Oncostatin M: an interleukin-6-like cytokine relevant to airway remodelling and the pathogenesis of asthma. Clin Exp Allergy. 2003; 33:1026–1032.
34. Simpson JL, Baines KJ, Boyle MJ, Scott RJ, Gibson PG. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Exp Lung Res. 2009; 35:781–794.
35. Sánchez-Guerrero I, Vegara RP, Herrero N, García-Alonso AM, Luna A, Alvarez MR. Cytokine serum profiles in allergic and non-allergic asthma. Increased production of IL-10 by non-allergic asthmatic patients. Allergol Immunopathol (Madr). 1997; 25:98–103.
36. Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S, Ishida S, Hiwada K. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995; 151:1354–1358.
37. Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001; 125:177–183.
38. ten Brinke A. Risk factors associated with irreversible airflow limitation in asthma. Curr Opin Allergy Clin Immunol. 2008; 8:63–69.
39. Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003; 124:474–481.
40. Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, Crim C, Yates JC, Silverman EK, Coxson HO, Bakke P, Mayer RJ, Celli B. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012; 7:e37483.
41. Soler-Cataluña JJ, Cosío B, Izquierdo JL, López-Campos JL, Marín JM, Agüero R, Baloira A, Carrizo S, Esteban C, Galdiz JB, González MC, Miravitlles M, Monsó E, Montemayor T, Morera J, Ortega F, Peces-Barba G, Puente L, Rodríguez JM, Sala E, Sauleda J, Soriano JB, Viejo JL. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012; 48:331–337.
42. Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, Mistry V, Reid C, Parker D, Dodson P, Jenkins M, Lloyd A, Rugman P, Newbold P, Brightling CE. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012; 83:36–44.
43. Joos GF, O'Connor B, Anderson SD, Chung F, Cockcroft DW, Dahlén B, DiMaria G, Foresi A, Hargreave FE, Holgate ST, Inman M, Lötvall J, Magnussen H, Polosa R, Postma DS, Riedler J. ERS Task Force. Indirect airway challenges. Eur Respir J. 2003; 21:1050–1068.
44. Cockcroft D, Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009; 103:363–370.
45. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26:948–968.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download