Abstract
Purpose
Airway inflammation, bronchial hyper-responsiveness (BHR), and bronchodilator response (BDR) are representative characteristics of asthma. Because allergic rhinitis (AR) is a risk factor for asthma development, we evaluated these 3 characteristics in AR using measurement of fractional exhaled nitric oxide (FeNO), a methacholine challenge test (MCT), and impulse oscillometry (IOS).
Methods
This study included 112 children with asthma (asthma group), 196 children with AR (AR group), and 32 control subjects (control group). We compared pulmonary function parameters and FeNO levels among the 3 groups. The AR group was subdivided into 2 categories: the AR group with BHR and the AR group without, and again pulmonary function and FeNO levels were compared between the 2 subgroups.
Results
FeNO levels were more increased in the AR and asthma groups than in the control group; within the AR group, FeNO was higher in the AR group with BHR than in the AR group without. The BDR was more increased in the AR group than in the control group when percent changes in reactance at 5 Hz (Δ X5) and reactance area (Δ AX) were compared. In the AR group, however, there was no difference in Δ X5 and Δ AX between the AR group with BHR and the AR group without.
Conclusions
Reversible airway obstruction on IOS and elevated FeNO levels were observed in children with AR. Because elevated FeNO levels can indicate airway inflammation and because chronic inflammation may lead to BHR, FeNO levels may be associated with BHR in AR. IOS can be a useful tool for detecting lower airway involvement of AR independent of BHR assessed in the MCT.
Allergic rhinitis (AR) has been shown to be a risk factor for the development of asthma.1,2 Lower airway pathology of AR may represent the link between these 2 diseases.3 Airway inflammation, bronchial hyper-responsiveness (BHR), and bronchodilator response (BDR) are representative characteristics of asthma. These have also been evaluated in AR in order to assess the degree of involvement of the peripheral airways.4,11 BHR in a methacholine challenge test (MCT) in AR has been suggested as a possible predictor of progression of AR to asthma.4,11 In cases of perennial AR, BHR may affect up to 80% of patients.5,6 Fractional exhaled nitric oxide (FeNO) has been studied in AR as a useful tool for detecting airway inflammation.7,8
The reversibility of airway obstruction is considered a diagnostic criterion for asthma and is a useful tool for evaluating lower airway pathology.9 While reversibility is determined by an increase in forced expiratory volume in 1 second (Δ FEV1)≥12% after inhalation of a bronchodilator in an adult, such a criterion has not yet been established in children.10 There have been few studies on BDR in AR children. Two different definitions of BDR assessed by spirometry have been proposed in AR children: Δ FEV1≥7.53% and Δ FEV1≥12%.11,12
Impulse oscillometry (IOS) has emerged as a powerful tool for evaluating pulmonary function in children who have failed to undergo forced expiration testing or show small changes in pulmonary function.13 Given this sensitivity, it is also useful for the evaluation of mild asthma or eosinophilic bronchitis, neither of which may be detected by spirometry.14 To the best of our knowledge, there have been few studies evaluating BHR in AR using IOS or to evaluate BDR in AR using IOS.15
The aim of this study was to evaluate BDR in the AR, asthma, and control groups. Furthermore, we sought to delineate the relationship among BHR, airway inflammation, and BDR in the AR group using MCT, FeNO measurement, and IOS.
This study included a total of 340 children who visited Severance Children's Hospital between July 2006 and August 2011. The children were divided into 3 groups: those who had atopic asthma and AR (asthma group, n=112), those who had AR without asthma (AR group, n=196) and those who had neither asthma nor AR (control group, n=32). Atopic asthma was defined as a Δ FEV1≥12% in response to a short-acting bronchodilator or BHR in the MCT (PC20≤16 mg/mL) accompanied by any typical asthma symptoms, such as cough or dyspnea.16 Of the 196 children in the AR group, 24 had BHR in the MCT (PC20≤16 mg/mL) without any typical asthma symptoms, such as cough or dyspnea, and the remaining 172 did not have BHR in the MCT (PC20>16 mg/mL). The control group showed normal spirometric results but had neither a history of respiratory disease, parenchymal lung abnormalities, BHR, nor BDR. We excluded non-atopic subjects based on allergy screening tests as well as subjects with asthma and without AR. Subjects had no history of respiratory infection for at least 6 weeks prior to the evaluation. We excluded subjects who had taken inhaled or systemic corticosteroids or leukotriene antagonists within 8 weeks prior to the study. All subjects were instructed to discontinue their β2-agonist medications for at least 3 days prior to pulmonary function and methacholine challenge tests.
Serum total and specific immunoglobulin E (IgE) levels and peripheral blood eosinophil counts were measured, and skin prick tests were performed at the initiation of the evaluation. IOS and spirometry were performed at the second visit, with IOS being performed prior to spirometry in order to avoid any influence of forced expiratory maneuvers on airway function during breathing at rest. Finally, FeNO was measured before the MCT and the MCT was performed at the third visit because repetitive exhalations of methacholine could decrease the FeNO levels. This study was approved by the Institutional Review Board of Severance Hospital (Seoul, Korea).
Total and specific serum IgE levels were measured using the Pharmacia CAP assay (Uppsala, Sweden). A specific IgE test was performed for 6 allergens commonly encountered in Korea: Dermatophagoides pteronyssinus, Dermatophagoides farina, egg white, cow milk, German cockroach, and Alternaria alternata. Atopy was defined as IgE levels greater than 0.7 kU/L that were specific to more than 1 allergen or total IgE levels greater than 150 IU/mL. Atopy was also defined as a positive skin reaction to more than 1 out of the 12 common aeroallergens, including 2 types of dust mites, cat and dog epithelium, as well as mold and pollen allergens, such as Alternaria, Aspergillus, birch, oak, mugwort, Japanese hop, ragweed, and bermuda grass. A saline solution was used as a negative control and a 0.5% histamine HCL (hydrochloric acid) solution was used as a positive control. Wheal diameters were measured after 15 min; a positive reaction was defined as a wheal diameter greater than 3 mm.17 All subjects had atopy according to these criteria.
A Jaeger MasterScreen PFT (pulmonary function test) system (Jaeger Co, Wurzberg, Germany) was used. Flow-volume curves were obtained before and after bronchodilator (BD) inhalation according to the American Thoracic Society guidelines.18 The MCT was performed according to standardized procedures.16 Each child inhaled increasing concentrations of methacholine (0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5, 10, 25, and 50 mg/mL) nebulized by a dosimeter (MB3; Mefar; Brescia, Italy) until FEV1 was reduced by 20% from a post-nebulized saline solution value. BHR was expressed as the provocative concentration of methacholine causing FEV1 to decrease by 20% (PC20, mg/mL). This was calculated by linear interpolation of the log dose-response curve.
FeNO was measured using a CLD 88 (Eco Medics, Duernten, Switzerland) at a constant expiratory flow rate of 50 mL/s. The measurements were made according to the ERS/ATS guidelines.19 Since nitrate-rich foods could affect FeNO levels, all children refrained from eating nitrate-rich foods for 2 h before measurement of FeNO. The mean value of the 3 consecutive measurements was calculated and regarded as the actual value.
The Jaeger MasterScreen IOS system (Jaeger Co, Wurzberg, Germany) was used to perform impulse oscillometry. Measurements were made according to the ERS/ATS guidelines and repeated just before spirometry after bronchodilator administration.20 The system was calibrated through a single volume of air (3 L) at different flow rates, with a reference device (0.2 kPa/L/s). The machine was also calibrated to the air temperature and pressure of the saturated gas. The impulse generator produced brief pressure pulses at intervals of 0.2 sec. In this study, mean resistance (R) values were calculated over a measurement period of 60 sec at frequencies of 5 Hz (R5) and 10 Hz (R10). Reactance (X) values were measured at 5 Hz (X5). We also recorded reactance area (AX), an integrated response index for reactance developed by Goldman,20 which reflects the integral of the negative values of reactance from 5 Hz to the resonant frequency. R5 reflects obstruction in the total airways, R20 only reflects the large airways, and the difference between R5 and R20 (R5-R20) is a parameter of the small airway alone. X5 and AX also reflect the degree of obstruction in the peripheral airways.21
During IOS, children sat upright with their heads resting against the back of the chair. They used nose clips and were instructed to breathe quietly through a mouthpiece. To decrease the shunt compliance of the cheeks, an investigator stood behind the patient and supported both the cheeks and the chin with their hands. Observations did not show any artifacts caused by coughing, breath holding, swallowing, or vocalization. Three correct measurements were averaged at each time point. We used acceptable coherence values of ≥0.8 at 10 Hz.21
The values with a parametric distribution in the text and tables are expressed as mean±SD. Values with a non-parametric distribution are expressed as median with interquartile ranges. Patients' characteristics, spirometric results, FeNO levels, and IOS values were evaluated using analysis of variance (ANOVA) for comparison of the 3 groups, using t tests or Fisher's exact tests for comparison of 2 groups if the data were parametric and continuous, and using the Kruskal-Wallis test and the Mann-Whitney test if the data were non-parametric and continuous. If the data were categorical variables, we used chi-square tests. We also compared the AR group with BHR to that without. Correlation coefficients between FeNO and PC20 levels were calculated with Pearson product moment correlation.
A P value of <0.05 was considered statistically significant. The Statistical Package for the Social Sciences (version 18.0, SPSS Inc., Chicago, IL, USA) was used for all analyses.
The clinical characteristics of the patients in this study are shown in Table 1. The 3 groups did not differ in terms of age, sex, and height. Serum total IgE levels and blood eosinophil counts were significantly higher in the AR and asthma groups than in the control group (P<0.05). Skin tests were performed on 224 children. The frequency of positive skin reactions was higher in the AR and asthma groups than in the control group (P<0.05). FEV1, the FEV1/forced expiratory vital capacity (FVC), and forced expiratory flow between 25% and 75% (FEF25-75) were significantly lower in the asthma group than in the AR and control groups (P<0.0001). There were no significant differences in FEV1, FEV1/FVC, or FEF25-75 between the AR and control groups.
BHR of the 3 groups as assessed by the MCT and FeNO levels are shown in Table 2. BHR was observed in 96% of the subjects in the asthma group and 12% of those in the AR group; it was significantly higher in the asthma and AR groups than in the control group (P<0.0001 and P=0.031, respectively). FeNO levels was higher in the asthma group than in the AR group (P<0.0001) and in the AR group than in the control group (P=0.005). In the total subjects, FeNO levels significantly correlated with PC20 levels (r=-0.337, P<0.0001) (E-Table 1).
BDR parameters as determined by spirometry and IOS in the asthma, AR, and control groups are shown in Table 3. The Δ FEV1 and % change in FEF25-75 were higher in the asthma group than in the AR and control groups (P<0.0001). The difference in resistance, as measured in % change, between 5 and 20 Hz (Δ R5-R20) and the differences in R5 (Δ R5), R10 (Δ R10), X5 (Δ X5), and AX (Δ AX) were also higher in the asthma group than in the AR and control groups (the asthma vs. AR groups: Δ R5-R20, P=0.001; Δ R5, P<0.0001; Δ R10, P=0.006; Δ X5, P<0.0001; Δ AX, P<0.0001)(the asthma vs. control groups: Δ R5-R20, P=0.009; Δ R5, P=0.001; Δ R10, P=0.033; Δ X5, P<0.0001; Δ AX, P< 0.0001). The variables Δ X5 and Δ AX were the indices of BDR, which were more elevated in the AR group than in the control group (P=0.012; P=0.022).
Serum total IgE levels and blood eosinophil counts were significantly higher in the AR group with BHR than in the AR group without, as shown in E-Table 2 (P=0.026 and P=0.009, respectively). Skin tests were performed on 135 children of the AR group. There was no significant difference in positive skin reactions between the 2 groups. The AR group with BHR tended to have higher FeNO levels than the AR group without (P=0.08) (E-Table 2, Figure. A). FeNO levels significantly correlated with PC20 levels (r=-0.175, P=0.045) in the AR group (E-Table 1). Since Δ X5 and Δ AX were significantly higher in the AR group than in the control group, comparisons of these values were made between the AR group with BHR and the AR group without; however, no difference was statistically not significant (E-Table 2, Figure. B).
Our study compared pulmonary function parameters and FeNO levels between the AR, asthma, and control groups. It also compared these parameters between the AR group with BHR and the AR group without. We demonstrated for the first time that BDR parameters, as determined by IOS (Δ X5 and Δ AX), may detect mild reversible airway obstruction in children with AR that cannot be detected by spirometry. We also found elevated FeNO levels in the AR group and a tendency for FeNO to rise in the AR group with BHR than in the AR group without. When Δ X5 and Δ AX were compared, there was no significant difference between the AR group with BHR and the AR group without.
It has long been debated whether BHR correlates simply with BDR because pathologic mechanisms in asthma, such as airway remodeling, are very complicated.22-24 Regardless, BHR and BDR have been accepted to be clinically important, independent indices that represent the pathology of asthma. In this study, BDR assessed using IOS, Δ X5, and Δ AX, did not correlate with BHR. It appears that the use of IOS to assess BDR in evaluating early bronchial involvement in AR may offer more information than the use of BHR (as measured by the MCT) alone.
IOS systems are increasingly being used in the clinical setting to test for airway obstruction and BDR.13 Reactance at 5 Hz (X5) reflects the elastic recoil of the peripheral airway, while the AX is a composite index of reactance.21 As confirmed by our study, these parameters are more sensitive in detecting changes in pulmonary function than spirometric results.25 Although FEF25-75 has also emerged as a good predictor of mild airway hyperresponsiveness or variable airway obstruction in AR,26 it did not distinguish AR from control subjects in our study, even when AR subjects were subdivided into those with and without BHR (data not shown).
FeNO was significantly higher in the asthma and AR groups than in the control group; within the AR group, the AR group with BHR had higher FeNO levels than the AR group without, but this difference was not statistically significant. FeNO levels also showed a significant correlation with PC20 levels in both total and AR subjects, respectively. It appears that FeNO may correlate with BHR in AR. This finding is supported by many studies that have attempted to replace BHR in the MCT with FeNO.27,28 Chronic airway inflammation secondary to allergic sensitization has been associated with not only increased FeNO, but also BHR.29,30 This finding is also supported by our result indicating that elevated total serum IgE levels and blood eosinophil counts were elevated in asthma and AR subjects, especially in AR subjects with BHR.
One potential strength of this study was careful selection of subjects to eliminate confounders. We only included atopic children in all groups to eliminate confounding factors from baseline airway inflammation that might increase FeNO level and BDR.14,31 Additionally, we excluded children with asthma but without AR. We considered the likelihood that asthma develops under different mechanisms when AR is not present due to the complex relationship between asthma and AR.32
Our study has some limitations. Although we only selected atopic children in all groups, the control group showed lower levels of total serum IgE and fewer positive skin test reactions than the other groups. We did not consider different degrees of atopic sensitization among the 3 groups. Second, since we did not obtain follow-up data, we could not confirm the development of asthma. Third, since the MCT, which is used to measure BHR, was performed through forced expiration on spirometry, while BDR was assessed using IOS. The use of different diagnostic tools for these 2 parameters may have introduced a measurement bias. Therefore, further studies with a prospective design that evaluate BHR and BDR using IOS are needed to understand lower airway pathology in AR.
In conclusion, children with AR have mild reversible airway obstruction that can be detected using IOS, in addition to elevated airway inflammation as indicated by higher FeNO. The tendency toward higher FeNO levels in the AR group with BHR than in the AR group without BHR represents that FeNO may be a useful tool for assessing BHR in AR. Furthermore, Δ X5 and Δ AX can be useful parameters for assessing lower airway involvement in AR independent of BHR in the MCT. These conclusions support the concept of a united airway disease of asthma and AR.4
Figures and Tables
Figure
(A) Fractional exhaled nitric oxide (FeNO) in the control group, the allergic rhinitis (AR) group with bronchial hyper-responsiveness (BHR), the AR group without, and the asthma group. FeNO levels were higher in the asthma and AR groups than in the control group. The AR group with BHR tended to have higher FeNO than the AR group without (P=0.08). (B) BDR in reactance at 5 Hz (Δ X5) and reactance area (Δ AX) increased more in the asthma and AR groups than in the control group. Moreover, Δ X5 and Δ AX were not significantly different between the AR group with BHR and the AR group without.

Table 1
Subject characteristics
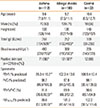
Table 2
The methacholine challenge test and measurement of fractional exhaled nitric oxide in the asthma, allergic rhinitis, and control groups

| Asthma (n=112) | Allergic rhinitis (n = 196) | Control (n=32) | |
|---|---|---|---|
| BHR (no [%]) | 107 (96)*† | 24 (12)* | 0 (0) |
| FeNO (ppb) | 50.4 (26.0/80.9)*† | 30.0 (16.3/49.8)* | 18.9 (12.3/38.2) |
Table 3
Bronchodilator response on spirometry and impulse oscillometry in the asthma, allergic rhinitis, and control groups

Data expressed as median (interquartile range).
*P<0.05 vs. control; †P<0.05 vs. allergic rhinitis.
FEV1, forced expiratory volume in 1 sec; FEF25-75, forced expiratory flow between 25% and 75%; R5-R20, difference in resistance between 5Hz and 20 Hz; R5, resistance at 5 Hz; R10, resistance at 10 Hz; X5, reactance at 5 Hz; AX, reactance area.
E-Table 1
Correlation between FeNO and PC20 levels in total and allergic rhinitis subjects.

| Correlation coefficient | Pvalue | |
|---|---|---|
| Total subjects (n=340) | -0.337 | <0.0001 |
| Allergic rhinitis subjects (n = 196) | -0.175 | 0.045 |
E-Table 2
Comparison of allergic rhinitis with and without BHR

ACKNOWLEDGMENTS
This study was supported by Korea Research Foundation Grant funded by the Korean Government (KRF-2010-0025171).
References
1. Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994; 15:21–25.
2. Leynaert B, Neukirch C, Kony S, Guénégou A, Bousquet J, Aubier M, Neukirch F. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004; 113:86–93.
3. Ciprandi G, Caimmi D, Miraglia Del Giudice M, La Rosa M, Salpietro C, Marseglia GL. Recent developments in United airways disease. Allergy Asthma Immunol Res. 2012; 4:171–177.
4. Braman SS, Barrows AA, DeCotiis BA, Settipane GA, Corrao WM. Airway hyperresponsiveness in allergic rhinitis. A risk factor for asthma. Chest. 1987; 91:671–674.
5. Townley RG, Ryo UY, Kolotkin BM, Kang B. Bronchial sensitivity to methacholine in current and former asthmatic and allergic rhinitis patients and control subjects. J Allergy Clin Immunol. 1975; 56:429–442.
6. Ciprandi G, Cirillo I, Vizzaccaro A, Tosca M, Passalacqua G, Pallestrini E, Canonica GW. Seasonal and perennial allergic rhinitis: is this classification adherent to real life? Allergy. 2005; 60:882–887.
7. Jouaville LF, Annesi-Maesano I, Nguyen LT, Bocage AS, Bedu M, Caillaud D. Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population-based sample of children. Clin Exp Allergy. 2003; 33:1506–1511.
8. Cardinale F, de Benedictis FM, Muggeo V, Giordano P, Loffredo MS, Iacoviello G, Armenio L. Exhaled nitric oxide, total serum IgE and allergic sensitization in childhood asthma and allergic rhinitis. Pediatr Allergy Immunol. 2005; 16:236–242.
9. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007; 120:S94–S138.
10. Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005; 60:13–16.
11. Capasso M, Varricchio A, Ciprandi G. Impact of allergic rhinitis on asthma in children: effects on bronchodilation test. Allergy. 2010; 65:264–268.
12. Suh DI, Lee JK, Lee JH, Koh YY. Bronchodilator response and its relationship to bronchial hyperresponsiveness in children with allergic rhinitis/asthma. Pediatr Allergy Respir Dis. 2010; 20:59–67.
13. Song TW, Kim KW, Kim ES, Park JW, Sohn MH, Kim KE. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008; 19:763–768.
14. Kim YH, Kim KW, Baek J, Park HB, Kim H, Song KJ, Lee JM, Sohn MH, Kim KE. Usefulness of impulse oscillometry and fractional exhaled nitric oxide in children with Eosinophilic bronchitis. Pediatr Pulmonol. 2013; 48:221–228.
15. Aronsson D, Tufvesson E, Ankerst J, Bjermer L. Allergic rhinitis with hyper-responsiveness differ from asthma in degree of peripheral obstruction during metacholine challenge test. Clin Physiol Funct Imaging. 2008; 28:81–85.
16. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975; 56:323–327.
17. Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979; 63:328–335.
18. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.
19. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.
20. Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther. 2001; 14:341–350.
21. Komarow HD, Myles IA, Uzzaman A, Metcalfe DD. Impulse oscillometry in the evaluation of diseases of the airways in children. Ann Allergy Asthma Immunol. 2011; 106:191–199.
22. Fruchter O, Hardak E, Yigla M. The response to bronchodilators in adults is not predictive of bronchial-hyperreactivity. J Asthma. 2009; 46:455–459.
23. Suh DI, Lee JK, Kim CK, Koh YY. Bronchial hyperresponsiveness to methacholine/AMP and the bronchodilator response in asthmatic children. Eur Respir J. 2011; 37:800–805.
24. Petanjek BB, Grle SP, Pelicaric D, Vrankovic D. Bronchodilator response in patients with persistent allergic asthma could not predict airway hyperresponsiveness. Allergy Asthma Clin Immunol. 2007; 3:123–127.
25. Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol. 2012; 129:671–678.
26. Ciprandi G, Signori A, Cirillo I. Relationship between bronchial hyperreactivity and bronchodilation in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2011; 106:460–466.
27. Ciprandi G, Tosca MA, Capasso M. Exhaled nitric oxide in children with allergic rhinitis and/or asthma: a relationship with bronchial hyperreactivity. J Asthma. 2010; 47:1142–1147.
28. Motomura C, Odajima H, Tezuka J, Murakami Y, Moriyasu Y, Kando N, Taba N, Hayashi D, Okada K, Nishima S. Effect of age on relationship between exhaled nitric oxide and airway hyperresponsiveness in asthmatic children. Chest. 2009; 136:519–525.
29. Patelis A, Gunnbjörnsdottir M, Malinovschi A, Matsson P, Onell A, Högman M, Alving K, Janson C. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012; 130:397–402.e2.
30. Steerenberg PA, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, Opperhuizen A, Brunekreef B, van Amsterdam JG. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003; 58:242–245.
31. Hervás D, Milán JM, Garde J. Differences in exhaled nitric oxide in atopic children. Allergol Immunopathol (Madr). 2008; 36:331–335.
32. Koh YY, Kim CK. The development of asthma in patients with allergic rhinitis. Curr Opin Allergy Clin Immunol. 2003; 3:159–164.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download