Abstract
A significant-source of allergens come from house dust that contain particles derived from arthropods, molds, and pet dander. This study evaluated mite and booklouse fauna from vacuumed dust samples in Beijing China (a temperate zone). Our survey was carried out in Beijing in the homes of mite allergic patients who visited our Allergy Department. In total, 38 homes were selected for the collection of dust samples by vacuuming, from December 2008 to January 2010. The flotation method was used to isolate mites from house dust. Permanent slides were prepared for mite specimens and mites were identified and counted under a microscope. In total, 1,798 separate mite and insect specimens were found in 345 dust samples taken from 38 homes. A total of 95 individual Dermatophagoides (D) siboney were detected in 35 dust samples from 19 homes (representing 5.3% of all mite and insect species found in house dust); in addition, this mite was found to co-exist with D. farinae (Hughes, 1961) in 33 dust samples. Our results demonstrated the presence D. siboney that co-existed with D. farinae in house dust in Beijing China (a temperate zone).
Dermatophagoides (D) siboney was first reported in Cuba. In a survey on Cuban domestic mites, Dusbábek et al.1 found that D. siboney represented 3.2%-40.1% of all mites detected in 6 samples collected from Havana and the Zapata peninsula. D. Pteronyssinus and Hirstia domicola were also observed in these dust samples; in addition, Malayoglyphus intermedius and Suidasia pontifica were identified in 4 cases. D. siboney was also reported in Puerto Rico by Montealegre et al.2; however, it has been only found in the Caribbean until now.
Surveys of allergic sensitization to D. siboney (conducted in several countries and regions) indicate it as a significant allergen source. Ferrándiz et al.3 reported that 148 adult Cuban asthmatic patients showed that the prevalence of a positive Skin Prick Test was high to D. siboney (88%) and IgE to D. siboney was found in 97% of patients. Castro et al.4 conducted a prick test on 232 Cuban allergic patients and reported an 88.4% rate of positive reactions to D. siboney, this suggests that sensitization to D. siboney is common. Casas et al.5 performed a comparative prick test study in Swedish and Cuban patients; subsequently, there was a higher reported rate of reactions to D. siboney in Sweden than in Cuba. However this mite is not present in Sweden and the results were explained due to a cross-reactivity with other Dermatophagoides species. The present study was conducted to evaluate if D. siboney can exist in Beijing China (a temperate zone). Simultaneously, we examined the house dust for other arthropod species because they also produce allergens.
This survey of dust mite diversity was conducted in several Beijing districts. The survey included the 38 homes of patients admitted to our allergy department, whose tests showed a positive prick test to mite allergen extracts from D. farinae and D. pteronyssinus and specific IgE (sIgE,d1 and d2) test ≥2 class (sIgE class: class 0:<0.35 kU/L, 0.35 kU/L≤ class 1<0.7 kU/L, 0.7 kU/L≤ class 2<3.5 kU/L, 3.5 kU/L≤ class 3<17.5 kU/L, 17.5 kU/L≤ class 4<50 kU/L, 50 kU/L≤ class 5<100 kU/L, class 6≥100 kU/L. ImmunoCAP 250 analysis system, Phadia, Uppsala, Sweden). The Institutional Review Board of Peking Union Medical College Hospital approved the study protocol. In agreement with Good Clinical Practice, all patients included in this study provided informed consent.
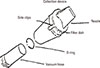
The collection equipment included a 1,200 W vacuum, an ALK Dust Trap (ALK, Copenhagen, Denmark) and a device to define 1 m2 of collection area or measuring the scale. The assembled collection device (ALK Dust Trap) was tightly connected to the vacuum hose with an O-ring (Fig. 1). One filter dish was used per dust sample. The nozzle was stored vertically to prevent the dust from falling out after the vacuuming was finished. The filter dish was carefully removed to prevent dust from spilling (Fig. 1). The dish was covered with a lid, sealed and labeled with ID that identified the place of collection. The nozzle was rinsed and dried before the placement of a new filter dish for the collection of the next sample.
Collection sites were locations where mites easily survive and breed (pillows, quilts, sheets, cotton padded mattresses, mattresses, sofas, rugs and floors). Collections were made over a 1 m2 area for 3 minutes. Samples were collected from the whole surface area and the collection time was shortened to 2 minutes for places with a surface area of less than 1 m2 (such as pillows, sofas and rugs). All dust samples were collected and prepared by the same 2 persons.
Data recording included numbering the collection device, filter plate and collection place, and recording related information that included family living conditions. Dust samples were transported to the lab and mites were isolated immediately (or stored at -20℃ in a freezer for later isolation) after the collection of each sample.
A flotation method isolated the mite bodies from dust samples. Mite specimens were stored in 70% alcohol. For convenience in isolation and identification, permanent slides were prepared using Hoyer's Medium.
Prepared slides were observed under a microscope. Mite species were identified according to the morphology of mites described in Krantz & Walter's classification method (2009) and combined with related information.1,2,6 The identity of the specimens as D. siboney was confirmed by Professor Larry G Arlian (Department of Biological Sciences, Wright State University USA), Professor Alexis Labrada (BIOCEN, Cuba), and Dr. Enrique Fernández-Caldas (CBF LETI, Research Laboratories, Madrid Spain).
Summarize the number of samples with detectable mites, calculate positive rate: positive rate=positive sample number/total sample number×100%. Count the total number of mites isolated from dust sample (including live mites, dead mites and incomplete remains), and calculate mite density: mite density (individuals/g house dust)=total number of detected (individuals)/weight of isolated dust (g house dust).
This survey was conducted from December, 2008 through January 2010 and 345 dust samples were collected from 38 homes in the Beijing area. A total of 1,798 separate specimens of mites and insects were found in 345 dust samples taken from 38 homes (Table 1). Mites were detected in 64.6% of samples. A total of 95 individual D. siboney were detected in 35 dust samples from 19 homes (constituting 5.3% of all mites and insect species in house dust), this study was the first time that D. siboney was isolated and identified in China. D. siboney and D. farinae were detected simultaneously in 33 dust samples from 18 homes, D. siboney and D. pteronyssinus were found in 8 homes, and D. farinae D. pteronyssinus and D. siboney were found in 6 homes. Moreover, in several dust samples with D. siboney, we also detected D. microceras (Griffiths et Cunnington, 1971), Tyrophagus putrescentiae (Schrank, 1781), Aleuroglyphus ovatus (Troupeau, 1878), Blattisocius dentriticus (Berlese, 1887), Cheyletus sp., Histiostoma sp. and Tetranychidae gen. sp. The percentages of Dermatophagoid mites in Beijing house dust were D. farinae 69%, D. pteronyssinus 24%, D. siboney 6%, and D. microceras 1%.
Male adults: Length of body 205-262 µm. The shape of the propodosomal shield was similar to that of D. farinae, its posterior margin extended to the sides and surrounding scapular setae. The epimeral region sclerotized more weakly than that of D. farinae, but was stronger than D. pteronyssinus. Epimere I usually separated from each other more far or less, but did not fuse into the epimeral plate. The hysterosomal shield was small, but did not extend forward to the seta d2. Epimere III was short and not bent to a right angle. The aedeagus was long, thin and pointed, attaching to a triangular epimeral plate. The link and width of the first pair of legs was similar to (or slightly thicker) than the second pair. Leg III slightly was longer and thicker than Leg IV, with a length ratio between them of 1:1.14-1.29. The chaetotaxy of legs was the same as D. farinae.
Female adults: Length of body 256-334 µm. Length of propodosomal shield 1.9-2.2 times its width and significantly longer than that of D. farinae with a punctuation smaller than that of the latter. Cuticle among setae d2 and d3 with a transverse striation. The morphological characteristics of the genital pore and bursa copulatrix were similar to D. farinae, and the internal orifice of the bursa copulatrix was above the anal region and connected with bottle-shaped vestibule of spermatheca via a long and thin tube. The ratio of distance between the third pair of genital hair gp (g) to the distance from third pair of the genital hair to the posterior margin of the genital fold (a) was 1:2.3-2.8. The legs had similar characteristics to D. farinae.
There are 3 peaks for the average mite density (May-July, September-October, and December-January); the density level was the highest from September to October, then January/May, and the lowest level in March/November (Fig. 2).
Booklouse specimens were found in 29 dust samples in 21 homes. This represents 55.3% of all homes, and 8.4% of all dust samples. Booklouse specimens were found in pillows, sheets, bed pads, mattresses, sofas and carpets. Booklice were found in 12 bed pads (12/93, 12.9%), 4 sofas (4/34, 11.8%), 6 pillows (6/54, 11.1%), 3 mattress (3/52, 5.8%), 2 carpets (2/9, 22.2%), 1 sheet (1/28, 3.6%), quilt (0/46), floor (0/9). Table 2 shows the differences of mite and booklouse faunae in relation to housing characteristics.
Body yellowish to brownish colored, antenna, maxillary palp and tarsus pale-yellowish colored, compound eyes black-purplish colored.
Female: body length 0.87-1.16 mm, width of vertex 245-265 µm. Vertex of the head with medium to large sized tubercles and usually smaller than the alveoli of small fine hairs. The spindle-shaped areas were usually well defined. Compound eyes consisted of 6-7 ommatidia. Seta SI (humeral seta of pronotum) were about equal to but always less than twice the length of the small fine hairs on the lateral lobe of the pronotum, pronotal setae on lateral lobe absent. Mesosternal setae 6-8. One pair of lateral prosternal setae (in addition to setae on the anterior half) was present on the posterior half of the prosternum. Abdominal terga 3-7 annulate type. Common trunk of gonapophyses branched distally.
Body pale brownish color, flagellum of antenna brownish color, maxillary palp white and legs whitish brown color, compound eyes black-reddish color.
Body length: female 1.28-1.39 mm, male 0.81-0.90 mm; width of vertex: female 296-327 µm, male 221-238 µm. Lateral areoles of vertex of the head with medium sized tubercles. Compound eyes consisted of 8 ommatidia. Seta SI (humeral seta of pronotum) sturdy, PNS 3-4, almost lined on a transverse level. Mesosternal setae 8-10. Common trunk of gonapophyses branched distally.
Lafosse Marin et al.9 described various species of house dust mites that colonized mattresses in Martinique Islands. They found D. pteronyssinus in 100% of the mattresses, Blomia tropicalis in 95.9%, Cheyletus malaccensis in 69.9%, D. farinae in 2%, and D. siboney in 6%. In contrast, our study of Beijing house dust found D. farinae in 69%, D. pteronyssinus in 24%, D. siboney in 6%, and D. microceras in 1%. The reason for the difference is presumably that Beijing (a temperate zone) is in a very different climate from the Caribbean (tropical zone).
This survey of homes in Beijing indicated the presence of D. siboney, which had previously been reported only in Caribbean countries such as Cuba1 and Puerto Rico.2 Those countries are in the tropical zone with a hot and humid climate with a small seasonal variation. This is the first time that this mite species has been reported in an Asian temperate zone. There are several possible reasons why this mite is being reported only now. First, systematic domestic mite surveys have been finished in many other countries; however, they are only now being completed in China. Second, D. siboney is difficult to distinguish from other mites because its morphology and molecular biology are similar to D. farinae and D. microceras.10 Third, transportation in Beijing is rapid and convenient; in addition, it is an international city with frequent interactions with foreigners. It is therefore salient to determine if D. siboney is present in other places in China.
D. farinae was not detected in the Cuba survey where D. siboney was discovered. In our survey, both D. siboney and D. farinae were detected simultaneously in 33 dust samples from 18 homes, this indicated that D. farinae can co-exist with D. siboney. D. farinae and D. siboney have similar morphology and the identification of these 2 mites could be confused on a microscopic examination. Further studies should investigate the habitat and mutualism of different mites of the Dermatophagoides genus. A new study is required to determine the role of D. siboney in the pathogenesis of allergic diseases in the Beijing area. Our survey investigated 345 samples from 38 homes and found D. siboney in 19 homes; subsequently, this may represent a newly recognized allergenic mite in Beijing and is of importance to the allergist. If D. siboney is found to be prevalent in other regions of China, it will raise the question of if D. siboney extract should be used for immunotherapy in China.
Currently, 13 groups of allergens from D. siboney have been discovered,10 among them Der s 1, Der s 2, and Der s 3 have been purified.11,12 These 13 groups of allergens have a different IgE binding frequency. The values were high (80% and 91% respectively) for Der s 1 (25 kDa) and Der s 2 (14 kDa); however, the IgE binding frequency was only 30% for Der s 3 (30 kDa).11 D. siboney was shown to be closer to D. farinae and D. microceras in aspects of morphology and allergen specificity when compared to D. pteronyssinus. The partial sequence homology of Der s 1 and Der s 2 with corresponding allergens of D. farinae was higher than 95%.10,11,12,13 Therefore, future studies should use a protein component level to detect D. siboney rather than morphologic identification.
In our present study, the number of booklice was significant at 2.61%. Booklouse specimens were found in 29 dust samples taken from 21 homes (55.3% of all homes and 8.4% of all dust samples). Booklouse specimens were found in pillows, sheets, bed pads, mattresses, sofas and carpets. Bookshelves are the ideal habitat of the booklouse; however, the present data demonstrated that booklice can be present in many environments other than bookcases. They also produce allergens; simultaneously, booklice are known to be an important pest of the stored grains and host of Rickettsia. In future research, we will study booklouse faunae in relation to housing characteristics such as private houses, apartments, central heating, bookcases and rugs.
Figures and Tables
 | Fig. 2Seasonal prevalence of domestic mites in relation to temperature, relative humidity, and precipitation. |
Table 1
Composition of mite and insect species in house dust

Table 2
Differences of mite and booklouse faunae in relation to housing characteristics-remove veritical lines within the table

ACKNOWLEDGMENTS
We would like to thank Professor Larry G Arlian (Department of Biological Sciences, Wright State University USA), Professor Alexis Labrada and Barbara Fernández (BIOCEN), Dr. Naomi Cuervo (IEC, Cuba), and Dr. Enrique Fernandez-Caldas (Director, R&D, Laboratories LETI, S.L. Madrid, Spain) for the identification of Dermatophagoides siboney. We would also like to thank Professor Richard W. Weber (Department of Medicine, National Jewish Medical and Research Center, Denver, USA) for help in writing the manuscript. We thank Professor Thomas A E Platts-Mills (Asthma and Allergic Diseases Center, University of Virginia Health System, USA) for the careful reviews. We also thank the ALK-Abelló A/S Company (Denmark) for providing the house dust collector device and filter plate. This study was supported by the grants from the ministry of science and technology, China. (No. 200802001) and supported by National Science Foundation of China (No. 30671943).
References
1. Dusbábek F, Cuervo N, de la Cruz J. Dermatophagoides siboney sp: pyroglyphidae) a new house dust mite from Cuba. Acarologia. 1982; 23:55–62.
2. Montealegre F, Sepulveda A, Bayona M, Quiñones C, Fernández-Caldas E. Identification of the domestic mite fauna of Puerto Rico. P R Health Sci J. 1997; 16:109–116.
3. Ferrándiz R, Casas R, Dreborg S. Sensitization to Dermatophagoides siboney, Blomia tropicalis, and other domestic mites in asthmatic patients. Allergy. 1996; 51:501–505.
4. Castro Almarales RL, Mateo Morejón M, Naranjo Robalino RM, Navarro Viltre BI, Alvarez Castelló M, Ronquillo Díaz M, García Gómez I, Oliva Díaz Y, González León M, Rodríguez Canosa JS, Labrada Rosado A. Correlation between skin tests to Dermatophagoides pteronyssinus, Dermatophagoides siboney and Blomia tropicalis in Cuban asthmatics. Allergol Immunopathol (Madr). 2006; 34:23–26.
5. Casas R, Ferrándiz R, Wihl JA, Fernández B, Dreborg S. Biologic activity of Dermatophagoides siboney and Blomia tropicalis allergens in exposed and unexposed mite-allergic individuals. Effect of patient selection on the biologic standardization of mite extracts. Allergy. 1999; 54:392–396.
6. Krantz GW, Walter DE. A manual of acarology. 3rd ed. Lubbock (TX): Texas Tech University Press;2009. p. 34–43.
7. Li FS. Psocoptera of China. Beijing: Science Press;2002. p. 77–103.
8. Li ZH, Kučerová Z, Zhao S, Stejskal V, Opit G, Qin M. Morphological and molecular identification of three geographical populations of the storage pest Liposcelis bostrychophila (Psocoptera). J Stored Prod Res. 2011; 47:168–172.
9. Lafosse Marin S, Iraola V, Merle S, Fernández-Caldas E. Étude de la faune acarologique des matelas de l'île de la Martinique. Rev Fr Allergol Immunol Clin. 2006; 46:62–67.
10. Ferrándiz R, Casas R, Dreborg S. Cross-reactivity between Dermatophagoides siboney and other domestic mites. II. Analysis of individual cross-reacting allergens after SDS-PAGE and Western blotting inhibition. Int Arch Allergy Immunol. 1998; 116:206–214.
11. Ferrándiz R, Casas R, Dreborg S, Einarsson R, Bonachea I, Chapman M. Characterization of allergenic components from house dust mite Dermatophagoides siboney. Purification of Der s 1 and Der s 2 allergens. Clin Exp Allergy. 1995; 25:922–928.
12. Ferrándiz R, Casas R, Dreborg S. Purification and IgE binding capacity of Der s 3, a major allergen from Dermatophagoides siboney. Clin Exp Allergy. 1997; 27:700–704.
13. Ferrándiz R, Casas R, Dreborg S, Einarsson R, Fernández B. Crossreactivity between Dermatophagoides siboney and other house dust mite allergens in sensitized asthmatic patients. Clin Exp Allergy. 1995; 25:929–934.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download