Abstract
Purpose
Asthma is the most prevalent disease in India according to the national survey conducted by NFHS 2 in 1998-1999. Matrix metalloproteinase-2 (MMP-2), a collagenase encoded by the MMP-2 gene, degrades the type IV collagen and is responsible for inflammatory responses. This is a pilot study evaluating the role of MMP-2 -1306C/T promoter single nucleotide polymorphism (SNP) in asthma pathogenesis.
Methods
A case-control study was performed with a total of 824 adult subjects, including 410 adult asthmatics and 414 healthy controls from regions of North India. The MMP-2 -1306C/T polymorphism was genotyped by the Tetra-Primer Amplification Refractory Mutation System Polymerase Chain Reaction (Tetra-Primer ARMS PCR).
Results
Statistical analysis of the results for the MMP-2 -1306C/T polymorphism revealed an extremely protective role of the mutant T allele in asthma pathogenesis with OR=0.45, 95% CI (0.35-0.58) and P=0.000. The heterozygous CT genotype also conferred protection from asthma with OR=0.37, 95% CI (0.27-0.51) and P=0.000. The homozygous TT genotype was also significantly associated with asthma with OR=0.35, 95% CI (0.16-0.72) and P=0.002. Moreover, the polymorphism was significantly associated with all the phenotypic traits of the disease.
Asthma is a polygenic disease of the lungs involving a complex interplay of the genetic makeup of an individual and the environmental stimuli, characterized by wheeze, shortness of breath (SOB), cough, dyspnea and bronchial hyperresponsiveness (BHR) caused due to the bronchial inflammation.1,2
Over the last decade, the prevalence of atopic diseases such as asthma, dermatitis and allergic rhinitis has been on a rise globally, and understanding the mechanisms of onset and severity of allergy, has offered a great challenge to researchers and scientists worldwide, due to the complex interplay of genetic as well as environmental factors.3 According to the second National Family Health Survey (NFHS 2), conducted in the year 1998-1999, asthma is the most common disease in India, both in urban as well as rural areas, as compared to tuberculosis (TB), diabetes, malaria, thyroid and other diseases.
A large number of case-control studies with respect to various genes have been conducted in the recent past, all around the world, so as to investigate the role of the various cytokine gene polymorphisms associated with asthma. The results have been found to vary profoundly with differences in the asthmatic populations studied across the world, mostly not revealing similar significances, but leading to a definite conclusion that asthma is a complex polygenic disease, which certainly does not follow classical Mendelian pattern of inheritance.4 As a result, it makes it all the more crucial to identify the genetic makeup associated with the complexity of asthma.
Lung airways of asthma patients undergo structural changes termed as "remodeling", characterized by infiltration of Airway Smooth Muscle (ASM) cells by mast cells5,6,7 which results in BHR and exacerbation of asthma.6,8 ASM cells increase in size and number in asthma patients9 and play a major role in inflammation and influx of inflammatory cells.10,11 Bronchial epithelial cells as well as the ASM cells produce MMP-2, which control their proliferation in an autocrine fashion. The ASM cells are known to modulate inflammation, inflammatory cells influx and angiogenesis in asthma patients.12
Matrix metalloproteinases (MMPs) are zinc-dependent proteases that are involved in the breakdown of extracellular matrix (ECM) in the normal physiological processes13 of the body such as cell proliferation, tissue remodeling, reproduction, embryonic development, differentiation, angiogenesis, apoptosis and host defenses, while the dysregulation of these matrixins has been associated with the disease processes such as arthritis, encephalomyelitis, chronic ulcers, tumor invasion, cancer as well as inflammation.14
Matrix metalloproteinase-2 (MMP-2) is a 72 kDa gelatinase and collagenase encoded by the MMP-2 gene in humans, which degrades the type IV collagen of the basement membranes. MMP-2 also plays a key role in the regulation of vascularization, endometrial menstrual breakdown as well as inflammatory responses.15 The ECM provides mechanical support to the cells and is a complex network of various proteins such as collagens, fibronectins, laminins, cytokines and growth factors. MMPs proteolytic activity can degrade the ECM as well as provide signals to the embedded cells to react to the stimuli.16 Moreover, patients with asthma have been found to have enhanced MMP-2 and MMP-9 linked gelatinolytic activity in their sputum.17
A lot of research has been conducted worldwide to analyze the role of MMP-2 gene in various types of cancers in humans as well as murine models, from tumor invasion and metastasis point of view. However, no such study has been conducted till date, to determine the role of MMP-2 -1306C/T gene polymorphism in inflammatory processes such as asthma.
Thus, this is the first study detecting the role of MMP-2 -1306C/T gene polymorphism in asthma pathogenesis with the hypothesis that MMP-2 -1306C/T gene promoter polymorphism has a protective role in asthma, as by inhibiting the MMP-2 enzymatic activity, the inflammatory response as well as the degradation of the basement membranes in the lung airways will be inhibited, thereby protecting the lungs from asthma like conditions.
Ethical Clearance for conducting the study on human blood samples was granted by the "Ethics Committee, PGIMER, Chandigarh". The study was conducted strictly in accordance with the ethical guidelines for bio-medical research on human subjects proposed by the "Central Ethics Committee on Human Research ICMR-2000" and of those contained in the "Declaration of Helsinki". The selection of asthma patients was based on physician's diagnosis. However, only the patients fulfilling the criteria of Global Initiative for Asthma guidelines18 for diagnosis of bronchial asthma were recruited in the study.
This is the first case-control study conducted in India to evaluate the role of MMP-2 -1306C/T polymorphism in asthma pathogenesis by recruiting a total of 824 adult subjects. The patients were recruited from different states of North India such as Punjab, Haryana, Chandigarh, Uttar Pradesh, Himachal Pradesh, Uttaranchal, Jammu & Kashmir, Rajasthan and New Delhi. A total of 410 asthma patients visiting the Out Patient Department, Pulmonary Medicine, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, were enrolled in the study, out of which, 323 subjects were asthma patients with allergic rhinitis. Informed Consent was duly obtained from the asthma patients participating in the study, and a detailed proforma of the asthma patients with a complete questionnaire regarding the clinical symptoms of the disease, i.e. wheeze/whistling, cough, SOB, allergy, early morning or night symptoms, along with spirometry tests was assessed. Complete information of the patient regarding name, age, sex, history of the disease, occupation was taken into account (Table 1).
A total of 414 age-matched, normal and completely healthy controls were inducted in the study. Some of the healthy volunteers were blood donors at various blood donation camps, educational institutes, employee groups. Completely healthy control subjects with no history of asthma, rhinitis, eczema, allergic skin diseases or any other co-morbid illness were recruited in the study. Care was taken that both the asthma patients as well as the control subjects were free from any other systemic immune or inflammatory conditions.
Asthma patients with history of any other pulmonary ailment such as TB, Chronic Obstructive Pulmonary Disease, bronchitis and emphysema were excluded from the study. No Allergic Bronchopulmonary Aspergillosis patients were taken in the study. Any subject having a first degree relative with asthma or allergy has not been recruited as a control in the present study. Not only the respiratory or allergic skin disorders, any subject with other diseases such as diabetes, high blood pressure or with drinking and smoking habits have also not been included as controls in the study. Each control was first enquired for all of the above conditions at the time of taking their written informed consent and before the collection of blood samples.
Spirometer device Spiro 232 (PK Morgan, Rainham, Kent, UK) was used for plethysmography which was performed strictly in accordance with the British Thoracic Society/Association of Respiratory Technicians and Physiologists guidelines.19 The subjects were asked to relax, avoid any kind of exercise at least 30 minutes prior to the test and avoid smoking or using bronchodilator at least 4 hours prior to the test. After placing the mouth-piece in the subject's mouth, the procedure involved a maximal forced expiratory and then a forced inspiratory manoeuvre. Three acceptable manoeuvres, all within 5% of each other were recorded as flow-volume curve.
The frequency of asthma patients with (60%-80%) FEV1/FVC observed % as well as the (40%-59%) FEV1/FVC observed % was the highest for the MMP-2 -1306 homozygous wild CC genotype with 61.6% and 13.2% respectively, and the lowest for the homozygous mutant TT genotype with 2.6% and 0% respectively. However, a limitation of the study is that since the spirometry of the control subjects was not conducted, statistical analysis could not be performed.
Total serum IgE concentration (IU/mL) was assessed for 219 asthma patients and 150 control subjects with an ELISA reader (Table 1). The bronchoalveolar lavage fluid (BALF) of subjects was examined for acid fast bacillus, aspergillosis and malignancy so as to distinguish the asthma patients from patients suffering from TB, allergic bronchopulmonary aspergillosis and lung cancer, respectively. However, the Th1/Th2 cytokine profiling of BALF was not performed. No significant difference for IgE level among cases and controls was observed for the polymorphism.
Body Mass Index (BMI), a measure of body fat based on height (cm) and weight (kg) that applies to adult men and women was calculated using the measures set by the U.S. National Heart Lung and Blood Institute (NHLBI).20 BMI of 190 asthma patients has been recorded in Table 1. The mean values have been calculated for 4 different categories: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2) and obesity (≥30.0 kg/m2). Apart from BMI (kg/m2), Height (cm), Weight (kg) and Body Surface Area (BSA) (m2) have also been recorded for the patients and have been given as mean values in Table 1.
The underweight, normal weight, overweight and obesity BMI categories had the highest prevalence of the homozygous wild CC genotype with 74%, 70.9%, 84.1%, and 70% respectively, and the lowest prevalence of the homozygous mutant TT genotype with 6%, 0%, 4.5%, and 0% respectively, in each category.
Blood samples were collected in EDTA coated vials and stored at -80℃ until genomic DNA extraction was done. Genomic DNA was isolated from the thawed blood samples by the Sodium Saline Citrate Buffer Method21 and checked for DNA on 0.8% agarose gel by electrophoresis.
Genotyping of the MMP-2 -1306C/T SNP was carried out by the Tetra-Primer ARMS PCR method as described previously.22 It is a rapid and sensitive high-throughput assay for the simultaneous detection of both the alleles in a single PCR using a set of four primers to amplify a larger fragment of DNA with the SNP and the amplicon representing each of the 2 alleles of the gene,23
Outer forward 5'-ACCAGACAAGCCTGAACTTGTCTGA-3', Outer reverse 5'-TGTGACAACCGTCTCTGAGGAATG-3', Inner forward 5'-ATATTCCCCACCCAGCACGCT-3' and Inner reverse 5'-GCTGAGACCTGAAGAGCTAAAGAGTTG-3'.
PCR was carried out in a thermal cycler (MyCycler, Bio-Rad Laboratories, Hercules, CA, USA) in a total volume of 25 µL containing: 10X PCR Buffer, 3 mM MgCl2, 1 mg/mL nuclease free BSA, 50 pmol of each set of primers, 10 mM of each dNTP, 0.125 U Taq polymerase and 2 µL genomic DNA. The PCR conditions were: initial denaturation at 94℃ for 5 minutes, followed by 35 cycles at 94℃ for 30 seconds, 60℃ for 30 seconds, 72℃ for 1 minute, and final extension step at 72℃ for 10 minutes. The results were observed by electrophoresis on 2% agarose gels stained with ethidium bromide and visualized by UV transillumination. The 542 bp and 379 bp products in a lane indicated the presence of the wild (C) allele, while 542 bp and 211 bp products in a lane marked the presence of the mutant (T) allele. Heterozygotes were observed as 542, 379, and 211 bp products in a lane (Figure).
The European Molecular Genetics Quality Network good practice guidelines have been followed. A few PCR vials with all the PCR contents except the DNA, were also included per PCR batch as "negative controls". No contamination was observed and there were no "false positives". To minimize the risk of contamination, sterilized and autoclaved solutions and equipment were used during DNA isolation. The ingredients for PCR were well stored at -20℃ and were thawed just before use.24 Retyping of samples was done at random to check for the homology of results.
The allelic distribution of the MMP-2 -1306C/T polymorphism between the asthma patients and healthy control subjects were analyzed statistically using Chi-square (χ2) test. The data was analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) and Epi Info version 3.4.3 (CDCP, Atlanta, GA, USA). Fisher's exact test was used wherever applicable. Statistical significance was assumed for P<0.05.
In the present study, a total of 824 subjects, including 410 adult asthma patients and 414 adult healthy controls were genotyped for the MMP-2 -1306C/T polymorphism. Very interesting results were observed for the MMP-2 -1306C/T gene polymorphism in the current study. Statistical analysis of the allelic frequencies clearly indicated the protective effect of the polymorphism with an increased presence of the mutant (T) allele among the healthy control subjects (27.8%) than in the asthma patients (14.8%) with OR=0.45, 95% CI (0.35-0.58) and P=0.000, conferring significant protection from asthma (Table 2).
The genotypic frequencies revealed that the homozygous wild genotype (CC) was more prevalent in asthma patients (73.7%) than in the controls (50.7%), while the heterozygous genotype (CT) was more prevalent among the controls (43.0%) as compared to the asthma patients (23.2%) with OR=0.37, 95% CI (0.27-0.51) and P=0.000, conferring significant protection from asthma. The homozygous mutant genotype (TT) was also more prevalent in the controls (6.3%) as compared to the asthma patients (3.2%) with OR=0.35, 95% CI (0.16-0.72) and P=0.002, again conferring significant protection from the disease. Moreover, it was observed that the CT+TT genotypic combination also conferred significant protection from the disease with OR=0.37, 95% CI (0.27-0.50) and P=0.000.
Further categorizing the asthma patients on the basis of the phenotypic characteristics of the disease (Table 3), as obtained from their detailed proforma, such as sex (male/female), occurrence (seasonal/throughout), severity (wheeze on exertion/wheeze at rest), family history (positive/nil), rhinitis (positive/nil), allergy to at least 2 provoking factors (positive/nil), smoking status (non-smoker/ever-smoker), longstanding cough (positive/nil), sputum production (positive/nil) and pattern of daily symptoms (morning/night SOB/anytime SOB), highly significant protective association was observed between the MMP-2 -1306C/T gene polymorphism and all of the asthma phenotypic traits (all P-values<0.05).
Taking into account, the genotypic distribution for each phenotypic trait, it was observed that for the trait of sex, the homozygous wild CC (56.6%) and the heterozygous CT genotypes (53.7%) were more prevalent among the females, while the homozygous mutant TT genotype was more prevalent among the males (61.5%). For the phenotypic trait of occurrence, the CC wild genotype (68.5%) as well as the CT heterozygous genotype (72.6%) was more prevalent among the throughout asthmatics while the mutant TT homozygotes (53.8%) were more prevalent among the seasonal asthmatics. For severity, it was observed that the prevalence of all the three genotypes, wild CC (53.0%), heterozygous CT (50.5%) as well as the TT (61.5%) mutant genotypes was higher in asthma patients with wheeze on exertion as compared to their frequencies in patients with wheeze at rest. It was further observed that patients with nil family history showed an increased presence of wild CC (66.6%), heterozygous CT (76.8%) and mutant TT (84.6%) genotypes as compared to their distribution among the patients with positive family history. Also the non-smoker asthmatics had a higher prevalence of wild CC (85.1%), heterozygous CT (84.2%) and mutant TT (61.5%) genotypes than the ever-smokers. On the contrary, asthmatics with positive rhinitis status had a higher prevalence of wild CC (77.8%), heterozygous CT (81.8%) and mutant TT (84.6%) genotypes as compared to the asthma patients with nil rhinitis. Similarly the allergic asthmatics had a higher prevalence of wild CC (89.1%), heterozygous CT (88.4%) and mutant TT (100%) genotypes than the asthmatics with no allergy. Moreover the patients with longstanding cough had higher frequencies of wild CC (82.1%), CT (78.9%) and mutant TT (100%) genotypes than the asthmatics with no cough. Furthermore, the sputum positive patients also showed greater prevalence of wild CC (76.5%), CT (77.9%) and mutant TT (76.9%) genotypes as compared to the sputum nil patients. Asthma patients with morning/night SOB pattern had a higher distribution of wild CC (77.2%), CT (74.7%) and mutant TT (61.5%) genotypes as compared to the anytime SOB pattern (Table 3).
Worldwide, research has been carried out to investigate the role of MMP-2 gene in various carcinomas. However, no study till date was conducted to evaluate the role of this gene in inflammatory processes such as asthma. Therefore, this novel study aimed to evaluate the association of MMP-2 -1306C/T SNP with asthma in a North Indian population, and striking results were obtained thereof.
The MMP-2 -1306C/T promoter region polymorphism had an extremely protective role in asthma which can be attributed to the fact that the basic function of the MMP-2 is to breakdown the basal laminas, degrade the ECM as well as to cause inflammation. MMPs can be post-transcriptionally controlled by Tissue Inhibitors of Metalloproteinases (TIMPs) which are tightly regulated so as to maintain the stable ratio of proteolysis and anti-proteolysis, as any deviation of this ratio from equilibrium results in remodeling,25 which is as such a key feature of lung airways of asthma patients.5,6,7
The present research is the first study of its kind that aimed to investigate the role of MMP-2 -1306C/T gene polymorphism in asthma pathogenesis with the hypothesis that any polymorphism in the MMP-2 gene will result in the production of a non-functional or less efficient MMP-2 protein entity that will lack the collagenase and gelatinase enzyme activity, which will further not be able to cause basement membrane degradation and inflammation, thereby reducing asthma symptoms.
The results obtained from the current study supported the above hypothesis with the observations that both the allelic as well as the genotypic frequencies revealed a highly protective role of the polymorphism in asthma. In the overall scenario, the wild C allele was more prevalent in the asthma patients (85.2%) than in the controls (72.2%) in contrast to the mutant T allele which was more prevalent among the control subjects (27.8%) than in the asthma patents (14.8%), indicating a highly protective role of the MMP-2 -1306C/T polymorphism in asthma propensity. It was also observed that the control individuals with at least one copy of the mutant allele (CT+TT), had a significantly decreased risk of asthma and were highly protected from the disease (Table 2).
Moreover, the MMP-2 -1306C/T polymorphism was also significantly associated with all the phenotypic traits of asthma demonstrating the protective effect of the SNP.
Not much of literature is present to be compared with the studied polymorphism in association with asthma, however, the results of the present study are strengthened by the results from the research conducted on a murine model of asthma, wherein it has been suggested that inhibition of MMP-2 and MMP-9 prevents allergen induced asthma as both of these MMPs play a key role in airway hyperresponsiveness by causing infiltration of inflammatory cells.26 Lung airways of asthma patients undergo structural changes termed as "remodeling" which is characterized by ASM hypertrophy and infiltration of ASM by mast cells5,6,7 which results in BHR and exacerbation of asthma.6,8 ASM cells increase in size and number in asthma patients9 and play a major role in inflammation and influx of inflammatory cells.1,10 MMP-2 is a major ASM-derived MMP and it has been shown that treatment of ASM cells with collagen I and thrombin, synergistically activates pro-MMP-2 protein and enhances MMP-2 expression. It has been observed that thrombin is abundantly present in the BALF and sputum of asthma patients, and its level rises on exposure to allergen and consequent antigen challenge.27,28
The MT1-MMP-/MMP-2 cascade was studied in induced sputum and BALF of asthmatics, bronchiectasis patients and controls by means of Western immunoblotting, immunohistochemistry and in situ hybridization, wherein increased levels of soluble, activated and autocatalyzed MTI-MMP and MMP-2 were observed, which shows the active process of destruction in diseased lungs.29
MMP-2 gene has been extensively studied in cancer, which have also yielded similar findings. The results of the present study are in accordance and conformity to the other studies conducted on various types of cancer and MMP-2 gene, where either lack of the MMP-2 enzyme activity due to polymorphism or inhibition by MMP-2 inhibitors such as TIMPs, decreases the risk of cancer. In research conducted in America on murine models with pancreatic cancer, tumors from mice treated with BB-94, an inhibitor of MMP-2 were significantly smaller in size than from the non-treated mice.30 Another American research suggested that the upregulation of MMP-2 enhances pancreatic tumor cell invasion in humans.31 A German based study on pancreatic cancer progression suggested an important role of MMP-2 in tumor invasion and its inhibition by inhibitor Batimastat resulted in a reduction in cancer cells.32 Another German study also demonstrated the presence of elevated MMP-2 levels in pancreatic cancer tissue samples.33 Elevated levels of activated form of MMP-2 were also detected in pancreatic cancer tissues in a Japanese study.34 These inferences were supported by the results from a study conducted in the UK, where it was observed that MMP-2 was implicated in the invasive phenotype of pancreatic cancer.35 A research conducted in China on laryngeal squamous cell carcinoma strongly suggested that MMP-2 gene silencing by siRNA significantly lowered the tumor invasion.36
A previous study conducted on MMP-2 structure and function has reported that a C→T transition at nucleotide -1306 in the promoter region of MMP-2 gene, abolishes the Sp-1 binding site and ultimately leads to down-regulation of the MMP-2 gene expression.37 Thus lesser MMP-2 in circulation, fails to induce inflammation, thereby unable to exacerbate asthmatic conditions.
The present pilot study clearly identifies the functional MMP-2 gene as an increased risk factor for asthma and that the presence of MMP-2 -1306C/T gene polymorphism deviates the immunity of an individual towards protection and decreased risk of asthma.
Therefore the current genetic findings expect to throw some light on the role of MMP-2 gene in asthma and this study concludes that the MMP-2 -1306C/T promoter region polymorphism of the gene confers a significant protection from asthma in the studied North Indian population.
Figures and Tables
Figure
Tetra-Primer ARMS PCR products of MMP-2 -1306C/T polymorphism on 2% agarose gel. Lanes 2, 3, 4, 7, 9, 13, 15: homozygous wild CC (542 bp and 379 bp), lanes 1, 5, 10, 11, 12, 16: heterozygous CT (542, 379, and 211 bp), lanes 6, 8, 14: homozygous mutant TT (542 and 211 bp), lane 17: 100 bp ladder.

Table 1
Characteristics of the study population

*Spirometry test, Weight, Height, BSA and BMI have been recorded for 190 asthma patients and mean values in each category have been calculated. †IgE levels were confirmed for 219 asthma patients and 150 controls and given as average in IU/mL.
FVC, Forced Vital Capacity; FEV1, Forced Expiratory Volume in 1 second; n.d. not determined.
Table 2
Distribution of MMP-2 -1306C/T genotypic and allelic frequencies in asthma patients and controls
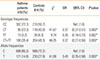
Table 3
Phenotypic characteristics and MMP-2 -1306C/T polymorphism

ACKNOWLEDGMENTS
J. Singh is thankful to the University Grants Commission, New Delhi, India, for providing grant support for the study (UGC Grant F.No. 40-161/2011 [SR]). It is submitted that the University Grants Commission, New Delhi, India, is a Government organization that funds studies in the Universities across the country to promote research, and is not affected by the outcomes of this manuscript in any way.
References
1. Holgate ST. Genetic and environmental interaction in allergy and asthma. J Allergy Clin Immunol. 1999; 104:1139–1146.
2. Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999; 402:B5–B11.
3. Sengler C, Lau S, Wahn U, Nickel R. Interactions between genes and environmental factors in asthma and atopy: new developments. Respir Res. 2002; 3:7.
4. Elston RC. The genetic dissection of multifactorial traits. Clin Exp Allergy. 1995; 25:Suppl 2. 103–106.
5. Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003; 167:78–82.
6. Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003; 167:1360–1368.
7. Jeffery PK, Laitinen A, Venge P. Biopsy markers of airway inflammation and remodelling. Respir Med. 2000; 94:Suppl F. S9–S15.
8. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998; 339:1194–1200.
9. Borger P, Tamm M, Black JL, Roth M. Asthma: is it due to an abnormal airway smooth muscle cell? Am J Respir Crit Care Med. 2006; 174:367–372.
10. Knox AJ, Pang L, Johnson S, Hamad A. Airway smooth muscle function in asthma. Clin Exp Allergy. 2000; 30:606–614.
11. Johnson SR, Knox AJ. Synthetic functions of airway smooth muscle in asthma. Trends Pharmacol Sci. 1997; 18:288–292.
12. Johnson S, Knox A. Autocrine production of matrix metalloproteinase-2 is required for human airway smooth muscle proliferation. Am J Physiol. 1999; 277:L1109–L1117.
13. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997; 378:151–160.
14. National Library of Medicine (US). Entrez gene: MMP2 matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) [Internet]. Bethesda (MD): National Library of Medicine;2013. cited 2013 Nov 30. Available from: http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4313.
15. Devarajan P, Johnston JJ, Ginsberg SS, Van Wart HE, Berliner N. Structure and expression of neutrophil gelatinase cDNA. Identity with type IV collagenase from HT1080 cells. J Biol Chem. 1992; 267:25228–25232.
16. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004; 16:558–564.
17. Cataldo D, Munaut C, Noël A, Frankenne F, Bartsch P, Foidart JM, Louis R. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000; 123:259–267.
18. Global Initiative for Asthma (US). Global Initiative for Asthma (GINA) guidelines [Internet]. [place unknown]: Global Initiative for Asthma;2013. cited 2013 Nov 30. Available from: www.ginasthma.org.
19. Guidelines for the measurement of respiratory function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med. 1994; 88:165–194.
20. National Heart, Lung, and Blood Institute (US). Calculate your body mass index [Internet]. Bethesda (MD): National Heart, Lung, and Blood Institute;2013. cited 2013 Nov 30. Available from: www.nhlbisupport.com/bmi/bmi-m.htm.
21. Roe BA, Crabtree JS, Khan AS. Protocols for recombinant DNA isolation, cloning, and sequencing. In : Roe BA, editor. Methods for DNA isolation. Hoboken (NJ): John Wiley & Sons;1996. Available from: www.genome.ou.edu/protocol_book/protocol_partIII.html.
22. Meijer MJ, Mieremet-Ooms MA, van Duijn W, van der Zon AM, Hanemaaijer R, Verheijen JH, van Hogezand RA, Lamers CB, Verspaget HW. Effect of the anti-tumor necrosis factor-alpha antibody infliximab on the ex vivo mucosal matrix metalloproteinase-proteolytic phenotype in inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:200–210.
23. Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001; 29:e88.
24. Xu J, Turner A, Little J, Bleecker ER, Meyers DA. Positive results in association studies are associated with departure from Hardy-Weinberg equilibrium: hint for genotyping error? Hum Genet. 2002; 111:573–574.
25. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–283.
26. Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999; 162:4212–4219.
27. Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, Kobayashi T, Hataji O, Urano H, Zhou H, Suzuki K, Adachi Y. Thrombin in the airways of asthmatic patients. Lung. 1999; 177:253–262.
28. Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med. 2004; 169:373–377.
29. Maisi P, Prikk K, Sepper R, Pirilä E, Salo T, Hietanen J, Sorsa T. Soluble membrane-type 1 matrix metalloproteinase (MT1-MMP) and gelatinase A (MMP-2) in induced sputum and bronchoalveolar lavage fluid of human bronchial asthma and bronchiectasis. APMIS. 2002; 110:771–782.
30. Zervos EE, Shafii AE, Rosemurgy AS. Matrix metalloproteinase (MMP) inhibition selectively decreases type II MMP activity in a murine model of pancreatic cancer. J Surg Res. 1999; 81:65–68.
31. Yang X, Staren ED, Howard JM, Iwamura T, Bartsch JE, Appert HE. Invasiveness and MMP expression in pancreatic carcinoma. J Surg Res. 2001; 98:33–39.
32. Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer. 2001; 93:204–211.
33. Gress TM, Müller-Pillasch F, Lerch MM, Friess H, Büchler M, Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995; 62:407–413.
34. Koshiba T, Hosotani R, Wada M, Miyamoto Y, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998; 82:642–650.
35. Bramhall SR, Stamp GW, Dunn J, Lemoine NR, Neoptolemos JP. Expression of collagenase (MMP2), stromelysin (MMP3) and tissue inhibitor of the metalloproteinases (TIMP1) in pancreatic and ampullary disease. Br J Cancer. 1996; 73:972–978.
36. Sun Y, Liu M, Yang B, Li B, Lu J. Role of siRNA silencing of MMP-2 gene on invasion and growth of laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2008; 265:1385–1391.
37. Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001; 276:7549–7558.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download