Abstract
Rifampin is commonly used as a first-line anti-tuberculosis drug, but it can induce a serum sickness-like reaction or anaphylaxis. However, it is possible for 1 drug antigen to induce 2 or more simultaneous immunologic reactions. Here, we report a case of a serum-sickness-like reaction and anaphylaxis induced concurrently by rifampin. A 25-year-old male presented with high fever and a maculopapular rash with vesicles on the hands, which developed 2 weeks following regular administration of anti-tuberculosis drugs for tuberculous meningitis, including rifampin. Elevated liver enzymes, peripheral neuropathy, and decreased serum C3 and C4 levels were found. Interestingly, these symptoms were accompanied by severe hypotension. A serum-sickness-like reaction was considered after excluding other potential causes for the fever. A drug provocation test showed that the fever developed after oral administration of rifampin, suggesting that rifampin was the cause of the allergic reaction. However, hypotension, epigastric discomfort, and diarrhea also accompanied these symptoms, indicating that IgE-mediated type I hypersensitivity could be part of the serum sickness-like reaction. An intradermal skin test clearly showed an immediate positive reaction to rifampin. This case was diagnosed as concurrent serum-sickness-like reaction and anaphylaxis induced by rifampin. One drug may therefore induce combined allergic reactions via 2 or more simultaneous hypersensitivity responses.
A drug allergy is defined as a harmful immunologic reaction to medication. Any drug can cause an allergic reaction corresponding to Gell and Coombs' type I, II, III, or IV hypersensitivity mechanisms. Drugs generally cause an allergic reaction by a single mechanism. Type I allergic reactions are mediated by drug-specific IgE and include urticaria, angioedema, and anaphylaixs.1 Type III is mediated by immune complex formation and complement activation, which includes serum sickness-like reactions (SSLR), drug fever, and vasculitis.1
However, a single drug antigen may induce 2 or more immunologic reactions simultaneously. The Aspergillus fumigatus antigen in patients with allergic bronchopulmonary aspergillosis can produce specific IgE antibodies through the type I hypersensitivity mechanism, and IgG and IgM precipitating antibodies through the type III hypersensitivity mechanism concurrently.2 Additionally, some bacterial or fungal antigens in patients with hypersensitivity pneumonitis can produce precipitating antibodies through a type III mechanism, and enhanced lung CD8+ T cells through a type IV hypersensitivity mechanism simultaneously.3
Here, we report a case of both SSLR and anaphylaxis induced simultaneously by rifampin, which was objectively diagnosed by skin and drug provocation tests.
A 25-year-old male was diagnosed with tuberculous meningitis at Chonnam National University Hospital, and was started on a treatment regime of 300 mg isoniazid (Yuhanizid®, Yu Han Pharm., Seoul, Korea), 800 mg ethambutol (Tambutol®, Chong Kun Dang Pharm., Seoul, Korea), 600 mg rifampin (Rifodex®, Chong Kun Dang Pharm.) and 1,500 mg pyrazinamide (Yu Han Pharm.) daily. He became nauseous, vomited, and had generalized abdominal discomfort 2 weeks later. He stopped taking the anti-tuberculosis drugs for 2 days, and the symptoms disappeared. He restarted taking the anti-tuberculosis drugs 2 days later but complained of pain and numbness in his extremities 30 minutes later. He was found to have conjunctival hemorrhage in both eyes, a maculopapular rash on the neck and anterior chest, and erythematous papules and vesicles on both hands.
He was admitted to the Department of Neurology. Laboratory tests revealed C-reactive protein, 10.9 mg/dL; erythrocyte sedimentation rate, 22 mm/h; white blood cells, 7,800/µL; eosinophils, 100/µL; hemoglobin, 15.8 g/dL; platelets, 230,000/µL; aspartate aminotransferase, 645 U/L; and alanine aminotransferase, 172 U/L. Levels of both C3 (62.9 mg/dL) and C4 (7.9 mg/dL) were low. Anti-nuclear antibody and anti-neutrophil cytoplasmic antibody were negative.
The anti-tuberculosis drugs were administered again. However, he developed a high fever of 39.1℃ 24 hours later, and his blood pressure decreased to 80/50 mm Hg. Pulse and respiratory rates increased to 120/min and 24/min, respectively. A maculopapular rash appeared on the neck and anterior chest and numbness and pain occurred in his extremities. A nerve conduction velocity test and electromyography showed bilateral, diffuse, axonal-type, and sensorimotor polyneuropathy. The first-line drugs were changed to second-line drugs, including 250 mg cycloserine (Closerin®, Dong-A Pharm., Seoul, Korea), 500 mg levofloxacin (Levofexin®, Il Dong-A Pharm.), and 1 g streptomycin (Chong Kun Dang Pharm.). Daily 50 mg prednisolone (Solondo®, Yuhan Pharm.) was added, and the symptoms and signs disappeared.
Because the first-line drugs were effective for treating the tuberculous meningitis, they were administered one at a time. Oral prednisolone was given continuously and then tapered off. Oral administration of 600 mg rifampin produced dyspnea, chest discomfort, and mild fever of 37.6℃ after 30 minutes. Blood pressure was 110/80 mm Hg, pulse rate was 132/min, respiration rate was 22/min, and oxygen saturation was 95%. The dyspnea and chest discomfort disappeared after 3 hours. Administration of 300 mg isoniazid or 800 mg ethambutol produced no symptoms. Administration of 1,500 mg pyrazinamide induced a fever of 38.3℃ after 8 hours. It was unclear whether the fever was due to the pyrazinamide or other causes related to the discontinuation of the oral steroid, because prednisolone was tapered off 1 day before administration of pyrazinamide. The drug responsible was not determined, and the patient was referred to the Department of Allergies for further evaluation.
Drug provocation tests with pyrazinamide and rifampin were repeated. Pyrazinamide was given orally at 1 hour intervals at doses of 5, 15, 25, 100, and 355 mg, but no reactions were observed. Thereafter, rifampin was given at doses of 6, 30, 90, and 270 mg. Gastrointestinal symptoms, such as diarrhea and epigastric discomfort, developed 30 minutes after the administration of 270 mg rifampin and lasted for 24 hours. A fever of 37.5℃ occurred 3 hours after the administration and was maintained for 3 hours. Blood pressure decreased to 90/60 mm Hg 5 hours after the administration and to 70/40 mmHg 3 hours later, but returned to the normal range 15 hours later. These results confirmed that rifampin was responsible for the adverse symptoms (Fig. 1). The levels of both C3 (76.0 mg/dL) and C4 (9.5 mg/dL) were below the normal range 5 days after the rifampin challenge. Unfortunately, the complement levels were not measured again after the symptoms disappeared.
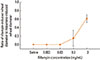
Rifampin skin tests were performed.4 Rifampin (600 mg) was dissolved in saline. The skin prick test was negative to 3 mg/mL, but the intradermal test showed an immediate positive wheal (8.0×7.6 mm) and flare reaction (22.2×22.0 mm) to 0.3 mg/mL. Saline and 1 mg/mL histamine were used as negative and positive controls, respectively. Intradermal tests to identify a non-irritating concentration (NIC) of rifampin were performed in nine normal controls. NIC was determined using the method described by Broz et al.,5 with some modifications. NIC was considered if the ratio of rifampin-induced mean wheal diameter to histamine-induced mean wheal diameter was less than 0.4. All test subjects were male, aged 22±1.58 (mean±SD) years. NIC was 0.3 mg/mL (Fig. 2).
Isoniazid, levofloxacin, ethambutol, and streptomycin were prescribed, but rifampin was not. No adverse drug reactions were observed. The tuberculous meningitis was finally cured without sequelae.
In this case, fever, skin rash, abdominal discomfort, abnormal hepatic function, and peripheral neuropathy occurred after a 2 weeks administration of rifampin. Decreased complement levels accompanied these symptoms, suggesting activation of the complex-mediated type III immune reaction. Rifampin-induced SSLR was considered, although other symptoms, such as arthralgia and lymphadenopathy, were not present. In particular, papules and vesicles were observed on both hands, which could be a cutaneous sign of SSLR.6 The occurrence of gastrointestinal disturbance,7 acute hepatitis,8 and neuropathy9 have also been reported in patients with SSLR. The rifampin provocation test induced the development of fever and a reduction in serum complement. It has been reported that rifampin can induce SSLR.10
However, the severe hypotension could not be explained by a SSLR diagnosis in this case. Hypotension can be a clinical manifestation of anaphylaxis. An intradermal skin test and drug provocation test showed that rifampin induced IgE-mediated allergic anaphylaxis. Thus, IgE-mediated anaphylaxis is another characteristic of the immune complex-mediated SSLR that induced the combined clinical manifestations of SSLR and anaphylaxis in this case. Rifampin has been reported to induce anaphylaxis.11
Namisato et al.12 and Luzzati et al.13 reported the possibility of combined type I and type III hypersensitivity reactions induced simultaneously by rifampin. They showed that flu-like symptoms and anaphylaxis developed simultaneously in patients taking rifampin. However, they did not provide any objective evidence in their case reports, such as decreased complement levels for a type III mechanism, or positive skin tests for a type I mechanism.
We documented that rifampin induced both SSLR and anaphylaxis through concurrent type III and type I hypersensitivity responses. Thus a single drug induced combined allergic reactions via 2 or more hypersensitivity responses simultaneously. It should be taken into consideration that mixed symptoms from multiple hypersensitivity reactions can occur in patients with a drug allergy. Further investigation is needed to identify the mechanisms involved.
Figures and Tables
References
1. Anderson JA. Allergic reactions to drugs and biological agents. JAMA. 1992; 268:2844–2857.
2. Safirstein BH, D'Souza MF, Simon G, Tai EH, Pepys J. Five-year follow-up of allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1973; 108:450–459.
3. Ando M. Type III and type IV allergy: summer-type hypersensitivity pneumonitis. Jpn J Med. 1989; 28:548–549.
4. Demoly P, Bousquet J, Romano A. In vivo methods for the study of allergy. In : Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, editors. Middleton's allergy: principles and practice. 7th ed. Philadelphia (PA): Mosby Elsevier;2009. p. 1267–1279.
5. Brož P, Harr T, Hecking C, Grize L, Scherer K, Jaeger KA, Bircher AJ. Nonirritant intradermal skin test concentrations of ciprofloxacin, clarithromycin, and rifampicin. Allergy. 2012; 67:647–652.
6. Lawley TJ, Bielory L, Gascon P, Yancey KB, Young NS, Frank MM. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984; 311:1407–1413.
7. Bielory L, Yancey KB, Young NS, Frank MM, Lawley TJ. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985; 13:411–417.
8. Perrillo RP, Pohl DA, Roodman ST, Tsai CC. Acute non-A, non-B hepatitis with serum sickness-like syndrome and aplastic anemia. JAMA. 1981; 245:494–496.
9. McLeod JG. Investigation of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1995; 58:274–283.
10. Parra FM, Pérez Elias MJ, Cuevas M, Ferreira A. Serum sickness-like illness associated with rifampicin. Ann Allergy. 1994; 73:123–125.
11. Harland RW, Lindblom SS, Munnell MO. Anaphylaxis from rifampin. Am J Med. 1992; 92:581–582.
12. Namisato M, Ogawa H. Serious side effects of rifampin on the course of WHO/MDT: a case report. Int J Lepr Other Mycobact Dis. 2000; 68:277–282.
13. Luzzati R, Giacomazzi D, Franchi F, Barcobello M, Vento S. Life-threatening, multiple hypersensitivity reactions induced by rifampicin in one patient with pulmonary tuberculosis. South Med J. 2007; 100:854–856.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download