Abstract
Eosinophilic fasciitis is a rare disease characterized by diffuse fasciitis with peripheral eosinophilia and progressive induration and thickening of the skin and soft tissues. We report a 19-year-old female who presented with pitting edema in both lower extremities. She had a history of excessive physical activity before her symptoms developed. Physical examination revealed 2+ pitting edema in both lower legs. She complained of mild pain in both knee joints and feet, with no tenderness or heating sensations. Laboratory results were unremarkable except for severe eosinophilia. Parasite infection, venous thrombosis, and cardiac and renal problems were excluded. A magnetic resonance imaging study of both lower extremities revealed increased signal intensity in the subcutaneous lesions, consistent with superficial inflammation of the fascia. Mixed perivenular lymphoplasmacytic and eosinophilic infiltration in the subcutaneous lesion were observed on biopsy. The patient was treated with corticosteroids, resulting in remarkable improvement in both edema and eosinophilia.
Eosinophilic fasciitis (EF), first described by Shulman in 1974, is a rare disorder of unknown etiology characterized by symmetric and painful swelling with progressive induration and thickening of the skin and soft tissues.1-3 In Korea, EF was first reported in 1980.4 Inflammation of the fascia is not associated with exposure to toxic chemicals or with collagen vascular disease. Peripheral eosinophilia is frequently present,5,6 and a good response to systemic corticosteroid therapy is characteristic of EF. In this case, we describe a 19-year-old female with EF whose only initial symptom was symmetric pitting edema in her lower legs without skin abnormalities.
A 19-year-old female visited our hospital with a 2 wk history of progressive pitting edema in both lower legs associated with a 2 kg weight gain. Symptom onset began shortly after intense physical exertion (dance practice). Her medical history included allergic rhinitis with sensitization to house dust mite and pollen, and she had visited the pediatric allergy clinic for about 10 years. None of her family members had a history of similar symptoms or specific diseases. Additionally, she noted mild pain in both knees and ankles, but tenderness was not remarkable on physical examination. She denied fever, dyspnea, chest pain, and urinary difficulty. She was not taking any medications. Physical examination revealed moderate bilateral pretibial pitting edema. No redness, induration, or thickened skin was observed. No contractures of joints were found, and muscle strength was normal in the upper and lower extremities.
At first, she visited the cardiovascular surgery clinic and was admitted for evaluation and treatment of deep vein thrombosis. However, computed tomography (CT) angiography of her lower extremities revealed no evidence of venous disturbance. Treatment with heparin and application of compression stockings did not alleviate her symptoms. Initial laboratory findings included a white blood cell count of 8,090 cells/µL with 28.9% eosinophils. Her eosinophil count was 2,340 cells/µL. Her hemoglobin level and platelet count were normal and her serum total IgE level was 786 kIU/L, but no remarkable change was observed compared to the previous year. The erythrocyte sedimentation rate and C-reactive protein level were not elevated. Serum urea nitrogen and creatinine were normal and urinalysis revealed no proteinuria or hematuria. Serum albumin was unremarkable at 4.2 g/dL. Serum protein electrophoresis results were within the normal range. Other laboratory data, including serum aminotransferase, alkaline phosphatase, cholesterol, and electrolyte levels, were normal. Further evaluations to determine the cause of her symptoms and peripheral eosinophilia were performed. There was no evidence of parasitic infection. A thyroid function test revealed normal thyroid function. The results of electrocardiogram and pulmonary function tests were normal. The patient was negative for rheumatoid factor and antinuclear antibody. An abdominal ultrasound showed no pathological changes. However, during the time of evaluation, the patient's blood eosinophil count increased rapidly to 4,880 cells/µL.
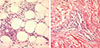
The patient was referred to our division of pediatric allergy considering the findings of peripheral eosinophilia and a past history of allergy, and a magnetic resonance imaging (MRI) was performed in her lower thigh. As shown in Fig. 1, the signal intensity of subcutaneous lesions was increased in fat-saturated T2-weighted images, suggesting inflammation of the subcutaneous fat layer and superficial fascia. Mild synovitis of both knees was also suggested. No evidence of thickening or fibrosis of the deep fascia was present. A biopsy of the right dorsal foot was performed, and the biopsy specimen included skin and underlying subcutaneous tissue. Biopsy examination revealed dermal edema and perivenular lymphoplasmacytic and eosinophilic infiltration in the subcutaneous fat tissue (Fig. 2). Based on the MRI and biopsy results, we diagnosed EF. We started intravenous corticosteroid therapy: dexamethasone 15 mg/day on the day of diagnosis. After 2 days of dexamethasone therapy, the symmetric pitting edema improved dramatically. The eosinophil count decreased rapidly from 4,880 to 140 cells/µL. We changed the intravenous dexamethasone to 30 mg/day of oral prednisolone, and the patient received an additional day of therapy with oral steroids. After a total of 3 days of intravenous plus oral steroid therapy, the patient recovered fully. She was discharged from the hospital without medication. Mild edematous changes in both legs developed 2 weeks later, but the patient's symptoms immediately relieved after administration of 30 mg/day prednisolone for 1day. She was monitored in an outpatient clinic for 4 months after cessation of therapy. The most recent eosinophil count was 320 cells/µL, and no relapses were observed.
When a young female presents with edema in both lower legs, common causes such as renal, cardiac, or venous problems should be considered. Here, we present a rare cause of symmetric pitting edema in the lower extremities. Two prior case reports have suggested EF as an unusual cause of generalized edema7 and pitting edema of the extremities.8 Common symptoms of EF include progressive thickening, redness, and hardness of the skin. The 'groove sign' of depressed veins suggests deep fascia involvement, which may be present in up to half of the patients with EF.9 The upper extremities are most often involved;5,9 however, our patient showed no cutaneous manifestations except for pitting edema, and her upper extremities were not involved. It was uncommon that our patient was a 19-year-old female, because EF occurs predominantly in young males, and only rarely in women and children.10,11 A recent history of intense physical activity or trauma has been reported in 30%-46% of patients.5,6,9 Considerable physical movement in our patient during dance practice may have acted as a disease trigger.
Although EF is characterized by unusual laboratory abnormalities such as peripheral blood eosinophilia, hypergammaglobulinemia and an elevated erythrocyte sedimentation rate, these laboratory findings do not always correlate with disease activity or prognosis.1,5 In the present case, almost all of the laboratory findings, including the serum protein electrophoresis results, were unremarkable. The only abnormal laboratory finding was severe blood eosinophilia. In addition, the blood eosinophil count increased rapidly during evaluation. There are no international diagnostic criteria for EF, although MRI and biopsy are accepted as diagnostic tools. Currently, full-thickness skin in a muscle biopsy is the gold standard for diagnosing EF, and a biopsy will typically reveal thickened fascia and inflammatory infiltrates composed of lymphocytes and eosinophils.3,5,9 Perivascular infiltrates of lymphocytes are almost always present, while eosinophil infiltrates are present in 69%-75% of cases.6,9 Several studies comparing MRI and biopsy results have demonstrated that MRI is useful for diagnosis of EF.12,13 Two cases in which MRI generated equal or superior results compared to those obtained with biopsy have been reported.13 Furthermore, MRI can be helpful in determining the response to therapy.12 In our case, MRI revealed inflammation of the superficial fascia without definite fibrotic changes. Neither the clinical manifestations of our patient nor the MRI findings revealed evidence of deep fascia or muscular involvement. The biopsy sample did not include deep fascia and muscle; however, consideration of the MRI results and biopsy findings of cellular infiltration of lymphocytes and eosinophils in subcutaneous lesions together was sufficient for the diagnosis of EF.
The clinical manifestations of EF can mimic other diseases, and the diagnosis of EF might be delayed in patients presenting with muscular pain because EF exhibits similarities to myopathies and eosinophilia-myalgia syndrome.14 Polyneuropathy or pulmonary disease or a history of L-tryptophan intake can distinguish eosinophilia-myalgia syndrome from EF. First, EF should be suspected when clinical symptoms and signs start to appear. For differential diagnoses, systemic sclerosis, scleroderma and mixed connective tissue disorders must also be considered.9 Hematologic disorders might be associated with EF, including thrombocytopenia, aplastic anemia and various lymphomas.3,15,16
Treatment of EF usually consists of systemic corticosteroids.9 In patients who do not respond to corticosteroids, immunosuppressive agents such as methotrexate, cyclosporin, hydroxychloroquine, and photochemotherapies have been used.9,17,18 In our case, a trial of short-term systemic corticosteroids was completely effective. The blood eosinophil count decreased dramatically, coinciding with an improvement in her symptoms; the change in the degree of eosinophilia was correlated with changes in symptoms. EF is usually benign, and 70%-90% of patients have been reported to experience a partial-to-complete response to corticosteroid therapy.5,9 Visceral involvement is extremely uncommon.9 Previous studies of pediatric patients with EF have suggested that a young age at the time of onset may be one of the prognostic factors for refractory fibrosis.6,11 It appears that EF with extensive skin involvement tends to advance to become a more severe disorder.11
In summary, although EF is an unusual disorder, it should be considered when a patient presents with eosinophilia and symmetric edema of the extremities. Normal laboratory findings cannot exclude a diagnosis of EF. The majority of patients are steroid responsive, and a proper diagnosis and therapeutic approach are critical for preventing long-term morbidity.
Figures and Tables
References
1. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–1039.
2. Oehling AG Jr, Walker C, Virchow JC, Blaser K. Correlation between blood eosinophils, T-helper cell activity markers and pulmonary function in patients with allergic and intrinsic asthma. J Investig Allergol Clin Immunol. 1992; 2:295–299.
3. Ciprandi G, Cirillo I, Vizzaccaro A, Milanese M, Tosca MA. Airway function and nasal inflammation in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2004; 34:891–896.
4. Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997; 99:178–184.
5. Tillie-Leblond I, Hammad H, Desurmont S, Pugin J, Wallaert B, Tonnel AB, Gosset P. CC chemokines and interleukin-5 in bronchial lavage fluid from patients with status asthmaticus. Potential implication in eosinophil recruitment. Am J Respir Crit Care Med. 2000; 162:586–592.
6. Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997; 27:3507–3516.
7. Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, Friend DS, Oettgen HC, Gerard C, Geha RS. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002; 109:621–628.
8. Blanchet MR, McNagny KM. Stem cells, inflammation and allergy. Allergy Asthma Clin Immunol. 2009; 5:13.
9. Rådinger M, Bossios A, Sjöstrand M, Lu Y, Malmhäll C, Dahlborn AK, Lee JJ, Lötvall J. Local proliferation and mobilization of CCR3(+) CD34(+) eosinophil-lineage-committed cells in the lung. Immunology. 2011; 132:144–154.
10. Daugherty BL, Springer MS. The beta-chemokine receptor genes CCR1 (CMKBR1), CCR2 (CMKBR2), and CCR3 (CMKBR3) cluster within 285 kb on human chromosome 3p21. Genomics. 1997; 41:294–295.
11. Lee JH, Chang HS, Kim JH, Park SM, Lee YM, Uh ST, Rhim T, Chung IY, Kim YH, Park BL, Park CS, Shin HD. Genetic effect of CCR3 and IL5RA gene polymorphisms on eosinophilia in asthmatic patients. J Allergy Clin Immunol. 2007; 120:1110–1117.
12. Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996; 2:449–456.
13. Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997; 185:785–790.
14. Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005; 280:13952–13961.
15. Shin HD, Kim LH, Park BL, Jung JH, Kim JY, Chung IY, Kim JS, Lee JH, Chung SH, Kim YH, Park HS, Choi JH, Lee YM, Park SW, Choi BW, Hong SJ, Park CS. Association of Eotaxin gene family with asthma and serum total IgE. Hum Mol Genet. 2003; 12:1279–1285.
16. Carlson CS, Eberle MA, Kruglyak L, Nickerson DA. Mapping complex disease loci in whole-genome association studies. Nature. 2004; 429:446–452.
17. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987; 136:225–244.
18. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.
19. Holland SM, Gallin JI. Disorders of granulocytes and monocytes. In : Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 15th ed. Vol. 1. New York (NY): McGraw-Hill;2001. p. 372.
20. Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001; 68:978–989.
21. Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987; 117:331–341.
22. Bugawan TL, Mirel DB, Valdes AM, Panelo A, Pozzilli P, Erlich HA. Association and interaction of the IL4R, IL4, and IL13 loci with type 1 diabetes among Filipinos. Am J Hum Genet. 2003; 72:1505–1514.
23. Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC, Li MD. A generalized combinatorial approach for detecting gene-by-gene and geneby-environment interactions with application to nicotine dependence. Am J Hum Genet. 2007; 80:1125–1137.
24. Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001; 69:138–147.
25. Brodie ED. Why evolutionary genetics does not always add up. In : Wolf JB, Brodie ED, Wade MJ, editors. Epistasis and the evolutionary process. New York (NY): Oxford University Press;2000. p. 3–19.
26. Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005; 175:5341–5350.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download