Abstract
Purpose
Long-acting β2 agonists (LABA) may mask ongoing bronchial inflammation, leaving asthmatic patients at greater risk of severe complications. The aim of this study was to compare the effect of combination therapy using low-dose inhaled corticosteroids (ICS) plus LABA on airway inflammation in asthma to the effect of medium-dose ICS alone.
Methods
Twenty-four patients with asthma not controlled by low-dose (400 µg per day) budesonide alone were enrolled in this prospective crossover study. Patients were randomized into 2 treatment phases: one receiving medium-dose (800 µg per day) budesonide (ICS phase), and the other receiving a combination therapy of low-dose budesonide/formoterol (360 µg/9 µg per day) delivered by a single inhaler (LABA phase). Each treatment phase lasted for 6 week, after which patients were crossed over. Asthma symptoms, lung function, and airway inflammation were compared between the 2 phases.
Results
Twenty-three patients completed the study; adequate sputum samples were collected from 17 patients. Asthma symptoms and lung function remained similar between the 2 phases. However, the mean sputum eosinophil percentage was higher in the LABA phase than in the ICS phase (5.07±3.82% vs. 1.02±1.70%; P<0.01). Sputum eosinophilia (≥3%) was more frequently observed in the LABA phase than in the ICS phase (six vs. two).
The goal of asthma treatment is to control and suppress clinical symptoms.1 Two primary treatment options for patients with moderate asthma inadequately controlled by low dose inhaled corticosteroids (ICS) are addition of long-acting inhaled β2 agonists (LABA), or increasing the dose of ICS administered.1,2 Several studies have shown that LABA in combination with low dose ICS results in better asthma control than medium dose ICS alone with respect to asthma symptoms, lung function, and the need for short acting β2 agonist rescue therapy.2-5 However, concerns regarding the safety of regular LABA use in asthmatic patients remain. LABA has been associated with higher risk of adverse outcomes, including severe exacerbations and deaths,6-11 although these events were relatively rare. These results suggest that LABA treatment may mask ongoing airway inflammation, leading to more severe asthmatic exacerbations, even with regular use of ICS. This study was designed to compare the effects of combination therapy (LABA plus low dose ICS) on airway inflammation in asthmatic patients to those of medium dose ICS therapy alone.
Eligible patients had a documented history of asthma treatment for more than 6 months at Samsung Medical Center. Patients at 16 to 70 years of age with a forced expiratory volume in one second (FEV1) of more than 60% of the predicted normal value were screened for enrollment. Each patient was assessed to establish his or her current treatment regimen, adherence to the current regimen, and level of asthma control. Asthma control was monitored using a simplified scheme, which included daytime symptoms, limitation of activity, nocturnal symptoms, need for reliever treatment, and lung function, as set forth by international guidelines.1 Patients were enrolled if their asthma was either uncontrolled, or only partly controlled by administration of low dose ICS (budesonide 200 µg, twice per day) for at least 3 months.
Patients were excluded if they had received treatment for acute asthma exacerbation within the previous 12 weeks, if they were smokers, pregnant, or lactating. All patients provided written informed consent prior to the study, which was approved by the Institutional Review Board of Samsung Medical Center.
This randomized, prospective, crossover study was carried out in an outpatient setting. Following an initial run-in period of 2 weeks, patients were randomly assigned to either of 2 treatment phases: one received medium-dose budesonide (400 µg twice per day; ICS phase), and the other received a combination therapy of budesonide/formoterol delivered by a single inhaler (160 µg budesonide plus 4.5 µg formoterol twice per day; LABA phase). Each treatment phase lasted for 6 weeks, followed by a 1-week washout period, after which patients were crossed over.
All patients completed asthma control test (ACT) questionnaires for their symptoms at the end of each treatment phase. The ACT contained 5 questions, each scored on a 5-point scale, with higher scores reflecting better asthma control.12 FEV1, peak expiratory flow rate (PEFR), and induced sputum eosinophil percentile were measured following completion of each treatment phase.
Sputum induction and processing were performed as described previously.13 Differential cell counts from sputum samples were recorded as the average of counts performed by 2 examiners on separate occasions in a blinded manner. Eosinophilic inflammation of airways was defined as an eosinophil percentage of ≥3% in the sputum.
The primary outcome was mean sputum eosinophil percentile following completion of each treatment phase. Secondary variables included FEV1, morning PEFR, and ACT scores.
Sample size was determined by assuming a standard deviation for repeated induced sputum eosinophil percentile of 0.69% for each patient. Thirty participants would therefore provide 90% power to detect a change of 2% or greater in sputum eosinophil levels. Due to recruitment difficulties, only 24 subjects were recruited, which reduced the power to 70%.
Statistical analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Individual parameters including FEV1, PEFR, ACT score, and eosinophil percentage in the induced sputum were compared using the Wilcoxon signed-rank test. All values are presented as means±standard deviation [SD]. Statistical significance was defined as P<0.05.
Twenty-four asthmatic patients were enrolled, with 23 patients (12 males and 11 females; mean age, 57.9 years; range, 34-68 years) initially randomized into either the LABA phase (12 subjects) or ICS phase (11 subjects); following completion of the initial study phase patients were crossed over into the alternative study phase. All subjects completed the study; however, adequate sputum for downstream analyses was obtained from only 17 patients (9 males and 8 females; mean age 54.5 years; range 35-68 years). No adverse events were reported during the study period.
Baseline measurements were collected following completion of the initial run-in period; mean FEV1 was 86.8±16.2% of the predicted value, morning PEFR was 371.0±75.5 L/min, and mean ACT score was 23.2±1.2 in the 23 subjects.
No differences in the 2 secondary outcomes, FEV1 and PEFR, were seen between the LABA and ICS phases. ACT scores were also similar in both phases (Table).
After 6 weeks of treatment, the mean sputum eosinophil percentage was 5.07±3.82% in the LABA phase, significantly higher than that seen in the ICS phase (1.02±1.70%, P=0.007). Sputum eosinophilia (≥3%) was present in 6 subjects during the LABA phase, compared to only 2 during the ICS phase (Fig. 2). Two patients showed a substantial increase in sputum eosinophil during the LABA phase (22.6% and 14.0%), which was decreased following completion of the ICS phase (0% and 3.66%).
When control of asthma is not achieved with low-dose ICS, the addition of a LABA is recommended, according to the Global Initiative for Asthma (GINA) guidelines.1 However, our results suggest that increasing the dosage of ICS provides better control of airway inflammation than addition of LABA, although asthma symptoms and lung function were comparable between the 2 strategies. Six of the 17 asthmatic patients had persistent airway eosinophilia during the LABA phase. Two patients showed a substantial increase in sputum eosinophil (more than 10%) using a combination of LABA plus low-dose ICS, which was decreased following treatment with medium-dose ICS alone. However, these changes in eosinophilic airway inflammation did not translate into differences in either lung function or asthma symptom scores between the ICS and LABA phases.
Traditional asthma management guidelines focus on symptoms, lung function, and the use of short acting β2 agonists rescue therapy.14 However, these features do not address underlying airway inflammation.15 Several studies have demonstrated that asthma treatment decisions which focus on reducing eosinophilic airway inflammation can reduce the risk of acute exacerbations.16,17
A recent meta-analysis of clinical trials found that use of LABA increased the likelihood of life-threatening asthma exacerbations and death, leading the United States Food and Drug Administration to issue warnings regarding regular use of LABA.18 Although the risk of adverse events is reduced with concomitant use of ICS, the overall safety of LABA remains controversial. A large-scale cohort study demonstrated a higher risk of severe exacerbations and hospitalization after combination therapy with LABA and ICS compared to increased dosage of ICS alone.11
LABA may worsen asthma symptoms as a result of continuous stimulation of β adrenergic receptors, leading to uncoupling and internalization of receptors, followed by a decrease in receptor density and receptor gene expression.7-9,19 In patients with uncontrolled asthma, the addition of LABA may also mask progression of inflammatory processes, allowing the disease to worsen before symptoms appear.6,7
To date, few studies have compared the anti-inflammatory effects of addition of LABA to those of medium-dose ICS alone. In this study, neither treatment strategy resulted in measurable clinical deterioration, despite differences in airway inflammation. However, it is uncertain whether these findings are the result of limitations of the present study, such as the short duration or the small number of patients enrolled.
In conclusion, a combination of LABA and low-dose ICS could result in masking of eosinophilic inflammation in some patients, compared to medium-dose ICS alone. These results suggest that before or during the step-up with LABA add-on therapy, an evaluation of airway inflammation may be necessary to prevent persistent airway inflammation. Further studies of the effects of airway inflammation on clinical outcomes are required.
Figures and Tables
Fig. 1
Study design. Following completion of the run-in period, patients began receiving either 800 µg budesonide per day (ICS phase) or 320 µg budesonide plus 9 µg formoterol per day (LABA phase) for 6 weeks, followed by a 1 week washout period, after which patients were crossed over.
FEV1, forced expiratory volume in one second; PEFR, peak expiratory flow rate; ACT, asthma control test; ISE, induced sputum eosinophil.
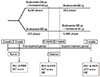
Fig. 2
Comparison of induced sputum eosinophil percentile between two phases.
ICS phase, 800 µg budesonide per day; LABA phase, 320 µg budesonide plus 9 µg formoterol per day; ISE, induced sputum eosinophil.

Table
Comparison of lung function and asthma symptoms between two phases

| Variables | ICS phase | LABA phase |
|---|---|---|
| FEV1 (% predicted) | 88.2±17.0 | 89.3±15.6 |
| PEFR (L/min) | 400.7±78.6 | 410.8±35.2 |
| ACT score | 24.36±0.81 | 24.45±0.69 |
No differences were observed between the ICS and LABA phases for FEV1, PEFR, and ACT score (P>0.05). Values are presented as means±SD.
ICS phase, 800 µg budesonide per day; LABA phase, 320 µg budesonide plus 9 µg formoterol per day; FEV1, forced expiratory volume in one second; PEFR, peak expiratory flow rate; ACT, asthma control test.
ACKNOWLEDGMENTS
This study was supported by a Samsung Medical Center Clinical Research Development Program grant [#CRS-107-56-2]. The investigators received no funding for the project.
References
1. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. United States: Global Initiative for Asthma (GINA);2010.
2. Greenstone IR, Ni Chroinin MN, Masse V, Danish A, Magdalinos H, Zhang X, Ducharme FM. Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database Syst Rev. 2005; (4):CD005533.
3. Kips JC, O'Connor BJ, Inman MD, Svensson K, Pauwels RA, O'Byrne PM. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med. 2000; 161:996–1001.
4. Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997; 337:1405–1411.
5. Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996; 153:1481–1488.
6. Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Effect of eight weeks of treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. Am J Respir Crit Care Med. 1994; 150:1006–1011.
7. McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. Am J Respir Crit Care Med. 1998; 158:924–930.
8. Aziz I, Wilson AM, Lipworth BJ. Effects of once-daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest. 2000; 118:1049–1058.
9. Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann Intern Med. 2004; 140:802–813.
10. Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2008; (3):CD006363.
11. Thomas M, von Ziegenweidt J, Lee AJ, Price D. High-dose inhaled corticosteroids versus add-on long-acting beta-agonists in asthma: an observational study. J Allergy Clin Immunol. 2009; 123:116–121.e10.
12. Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, Sohn SW, Park CH, Park HW, Kim SS, Cho SH, Min KU, Kim YY, Chang YS. Correlation between the Korean version of Asthma Control Test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008; 23:621–627.
13. Kwon NH, Oh MJ, Min TH, Lee BJ, Choi DC. Causes and clinical features of subacute cough. Chest. 2006; 129:1142–1147.
14. Sims EJ, Price D, Haughney J, Ryan D, Thomas M. Current control and future risk in asthma management. Allergy Asthma Immunol Res. 2011; 3:217–225.
15. Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med. 1998; 157:4–9.
16. O'Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol. 2011; 128:257–263.
17. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002; 360:1715–1721.
18. U.S. Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called Long-Acting Beta-Agonists (LABAs). Silver Spring (MD): U.S. Food and Drug Administration;cited 2012 Feb 18. Available from: http://www.fda.gov/Drugs/DrugSafety/postmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm.
19. Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006; 144:904–912.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download