Abstract
Purpose
Measurement of the fractional concentration of exhaled nitric oxide (FeNO) is a quantitative, noninvasive, simple, safe method of assessing airway inflammation. While FeNO measurement has been standardized, reference values for elementary school children are scarce. The aim of this study was to establish reference values for FeNO in children.
Methods
FeNO was measured in elementary school children at 6-12 years of age in Seoul, Korea, following American Thoracic Society guidelines and using a chemiluminescence analyzer (NIOX Exhaled Nitric Oxide Monitoring System, Aerocrine, Sweden). A total of 1,252 children completed a modified International Study of Asthma and Allergy in Children (ISAAC) questionnaire; FeNO was measured in 1,063 children according to the protocol and in 808 children defined as healthy controls.
Results
Mean FeNO were 10.32 ppb, 16.58 ppb, and 12.36 ppb in non-atopic, atopic, and all 808 healthy controls, respectively. FeNO was not associated with age and gender. The FeNO reference equations were determined by multiple linear regression analysis, taking into account the variables of age, height, weight, total IgE, eosinophil percent, and bronchial hyper-responsiveness (methacholine PC20). FeNO=0.776+0.003×total IgE+0.340×eosinophil percent; coefficient of determination (R2)=0.084 in the 501 healthy non-atopic controls. FeNO=-18.365+1.536×eosinophil percent, R2=0.183 in the 307 healthy atopic controls; and FeNO=-7.888+0.130×Height+0.004×total IgE+1.233×eosinophil percent, R2=0.209 in the 808 all healthy controls. Eosinophil percent was correlated with FeNO in all healthy controls. FeNO was not associated with BMI.
Asthma is a heterogeneous group of disorders, but the central process in the pathophysiology of asthma is airway inflammation. Nitric oxide (NO) is a marker of airway inflammation and is characterized by eosinophil activation. The fractional concentration of exhaled nitric oxide (FeNO) is increased in individuals with asthma and correlates with measures of eosinophilic inflammation in the airway mucosa.1 Measurement of FeNO would be a useful clinical tool to diagnose and follow up asthma because it is noninvasive, passive, inexpensive, and the result is immediately available. Also, in clinical practice, it is easily performed in school-age children.
However, the potential clinical value of FeNO measurement is limited due to a lack of standardization. Until now, there have been only a few studies of FeNO in healthy subjects.1 In addition, confounding factors, such as age, sex, and atopy, have not always been taken into account.2 Reported values of FeNO in healthy subjects vary from 3 to 88 ppb, a range that may be attributed both to different measurement techniques and small numbers of subjects studied.2 Increased use of FeNO measurement for diagnosing and monitoring of asthma requires reference values of FeNO. Understanding of the factors that have an effect on FeNO in healthy subjects is also a prerequisite for its use in clinical assessment of asthma. Previous population-based studies showed that FeNO had a close relationship with atopy in children and seemed to increase with age in healthy children.1 No gender differences have been reported.1
The primary objective of this study was to establish reference values of FeNO, according to international guidelines, in healthy children at 6-12 years of age and to add to the knowledge of the reference range for this age group. The secondary objective was to investigate the effect of possible confounders on FeNO measurement. We investigated the reference values of FeNO in a population-based sample of school-age children.
Between July and August of 2012, a total of 1,338 children were recruited from a population of students in an elementary school in Seoul, Korea. The prevalence of allergic diseases and their symptoms was determined by administering a modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire. The prevalence of parental history of physician-diagnosed allergic diseases, including atopic dermatitis, asthma, and allergic rhinitis, was 45.5% (Table 1). This study was approved by the Institutional Review Board of Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. Written informed consent was obtained from each parent or guardian following a detailed explanation of the study.
The modified Korean version of the ISAAC questionnaire was previously validated as a tool for assessing allergic symptoms and diagnosis in Korean children.3,4 The questionnaire consists of 3 main sections: 1) general characteristics, including name, gender, birth date, height, and weight; 2) history of symptoms related to asthma, allergic rhinitis, atopic dermatitis, allergic conjunctivitis, and food allergy; and 3) exposure to environmental factors associated with allergic disease. The questionnaire was completed by parents or guardians of the elementary school children who were enrolled.
White blood cell counts were obtained, and peripheral blood eosinophils were determined by a Coulter STKS (Coulter Electronics Inc., Hialeah, FL, USA). Serum total immunoglobulin E (IgE) was measured by a fluorescent enzyme immunoassay (ImmunoCAP system, Phadia AB, Uppsala, Sweden).
Skin prick tests (SPTs) for 19 allergens (D. pteronyssinus, D. farinae, cockroach, grass, alder, birch, oak, Japanese hop, Korean hop, mugwort, ragweed, dog, cat, Alternaria, Aspergillus, peanut, cow's milk, egg white, and soybean) were performed following the manufacturer's instructions.5 No children had taken antihistamines or other medications that could influence the results, for 7 days before the SPT. Allergen extracts (Allergy Therapeutics, Ltd., ATL, Bencard, England), along with the appropriate negative (normal saline) and positive (1 mg/mL histamine) controls, were applied on the forearm using the prick technique. Wheals were considered positive if they were greater than 3 mm in diameter.
Lung function data were collected at the children's school by trained field technicians using a portable microspirometer (Microspiro Hi-298, Chest Corporation, Tokyo, Japan) according to American Thoracic Society guidelines.6 Methacholine challenge tests were carried out using a modification of the method described originally by Chai et al.7 Bronchial hyper-responsiveness (BHR) was expressed as the methacholine concentration that caused a 20% decrease in FEV1 from baseline (PC20). Details are described elsewhere.8
FeNO measurements were performed according to American Thoracic Society (ATS) guidelines9 with a chemiluminescence analyzer (NIOX Exhaled Nitric Oxide Monitoring System, Aerocrine, Sweden).
Because of skewed data, analysis of FeNO values were performed with log-transformed data, and the results are presented as geometric mean and 95% confidence intervals (CIs). Data on the children's atopic characteristics and gender differences in FeNO values were compared using independent-sample t tests. One-way ANOVA was used to analyze age differences in FeNO values. Simple and multiple linear regression models were used to analyze factors affecting FeNO. All statistical analyses were performed using SPSS version 18.0. For all analyses, a P value of <0.05 was considered statistically significant.
A total of 1,252 children between 6 and 12 years of age completed the modified ISAAC questionnaire. FeNO measurements were performed in 1,063 children. WBC counts with the percentage of eosinophils and total serum IgE were available from 1,106 and 1,100 children, respectively. SPTs were carried out on 1,143 children, 1,243 completed pulmonary function tests, and 1,151 underwent a methacholine challenge test.
Subject characteristics, including the total study sample with FeNO measurements, are presented in Table 1. The mean age of the children was 9.21±1.75 years. There were 620 boys (49.8%) and 624 girls (50.2%). The mean BMI of the children was 17.96±3.19 kg/m2, with most children (80.3%) below the 85th percentile. Of the participants, 808 were included as healthy children according to the following exclusion criteria: physician-diagnosed asthma, wheezing during the last 12 months, premature birth (gestational age <37 weeks), low birth weight (<1,500 g), obesity (BMI >25 kg/m2), or currently taking medication for upper respiratory infection10 (Figure). Physician-diagnosed asthma included children who had answered "yes" to the question "Have you ever been diagnosed by a physician to have asthma?"
The 808 healthy participants were divided into 2 groups: 501 non-atopic and 307 atopic children. The mean FeNO measurements were 10.32 ppb in the non-atopic children, 16.58 ppb in the atopic children, and 12.36 ppb in all healthy control children. The upper 95% CIs were 12.69 ppb, 19.13 ppb, and 14.87 ppb in the non-atopic, atopic, and all healthy children, respectively. The mean FeNO was significantly higher in healthy atopic children than in healthy non-atopic children. The healthy atopic children were identified by using positive SPTs for 19 allergens. The reference values for FeNO are shown in Table 2.
We investigated whether differences in FeNO concentration were associated with BMI, eosinophil percentage, total IgE, and BHR. The simple linear regression analysis of factors affecting FeNO concentration is shown in Table 5. Eosinophil percentage and total IgE were both significantly correlated with FeNO levels in non-atopic, atopic, and all healthy children. Methacholine PC20 was associated with FeNO values in atopic and all healthy children. BMI was an independent variable affecting FeNO.
The FeNO reference equations were determined by multiple linear regression analysis, taking into account the variables of age, height, weight, total IgE, eosinophil percent, and bronchial hyper-responsiveness (methacholine PC20). FeNO=0.776+0.003×total IgE+0.340×eosinophil percent; coefficient of determination (R2)=0.084 in the 501 healthy non-atopic controls. FeNO=-18.365+1.536×eosinophil percent, R2=0.183 in 307 healthy atopic controls; and FeNO=-7.888+0.130×Height+0.004×total IgE+1.233×eosinophil percent, R2=0.209 in all 808 healthy controls. Eosinophil percent was correlated with FeNO in all healthy children. However, FeNO was not associated with BMI.
This study demonstrated the reference values of FeNO in a large population of "healthy" children at 6-12 years of age. The reference values of FeNO were geometric mean 12.36 (95% CI, 9.85-14.87) ppb in a group of 808 healthy children comprised of 501 who were non-atopic 10.32 (95% CI, 7.95-12.69) ppb and 307 who were atopic 16.58 (95% CI, 14.03-19.13) ppb. Consistent with prior results, we found a positive association between FeNO and atopy, as determined by the SPT for aero-allergens in these healthy young children. We measured FeNO at a constant flow rate of 50 mL/s and a constant mouth pressure of 17 cm H2O according to ATS and European Respiratory Society (ERS) recommendations.11 These results highlight the importance of standardized measurement methods in epidemiologic studies of FeNO.
This study with a larger sample demonstrated that FeNO was not associated with age and gender but positively associated with eosinophil percentage, total IgE, and height in healthy children. The study results are similar to those reported in Caucasian children.1,2,9,12,13
Several investigators have reported FeNO values in healthy subjects measured using equipment identical to that used in this study. A previous study established a regression equation for FeNO in 114 non-atopic, non-smoking healthy Caucasian children at 6.9-15.7 years of age in Finland. They reported a mean value of 10.3 ppb, which is close to the 12.36 ppb observed in our study.1 Kharitonov et al.14 reported a mean of 15.6±9.2 ppb in 20 healthy children at 7-13 years of age who did not have atopy or history of allergic diseases, and had normal lung function. The range of FeNO in our study is within the range reported in that study.14 Zhang et al.15 reported a median FeNO of 11 ppb (range, 8-16 ppb) in children from northern China. They included 300 healthy students at 6-14 years of age who had no atopy15 and reported similar mean FeNO value as ours. Buchvald et al.2 reported a mean FeNO of 9.7 ppb in a large sample of 405 healthy children without atopy and environmental tobacco smoke (ETS) exposure. Their exclusion criteria were history of asthma or related respiratory symptoms as defined by the ISAAC questionnaire.2 Again, their reported mean value of 10.5 ppb is similar to ours.
In this study, we did not find an association between FeNO and age. The reason that the FeNO level increased with age is not known, and an age-related FeNO trend has not yet been reported in adults.2,16 This age dependency in children may be related to developmental and maturational changes, increased lung volume and airway surface area,2,17 changes in airway NO diffusion coefficients (which may be dependent on surface area),2 or age-dependent induction of inducible nitric oxide synthase secondary to recurrent immunological stimulation.2 The use of a constant exhalation flow rate (50 mL/s) in children having different airway sizes may also be a factor explaining the age dependency of exhaled NO, because a constant flow rate would be relatively higher in young children.2 However, age was not associated with FeNO values in multiple linear regression analysis by height in this study. These results suggest that age dependency may present due to developmental changes, whereas height was a determinant in all healthy children.
In this study, we could not find any gender difference in healthy children. The positive association of male gender with FeNO levels is consistent with those of previous reports in healthy adults and healthy children.18 The mechanism of how gender affects FeNO levels remains unknown.18 A few hypotheses have been suggested, including differences in airway surface area and caliber, in flow in the mouth, within the intrathoracic airway at the same flow rate, and an effect of estrogen on NO synthase expression.18 However, Olin et al.18 demonstrated that gender was not independently associated with FeNO and that the association of gender with FeNO reported in previous studies was related to height. FeNO data should be interpreted taking the height of the child into account. In most studies,1,2,12,15 there were no significant relationships between BMI and FeNO values. We also observed that BMI was not correlated with FeNO in non-atopic, atopic, or all healthy children.
BHR is positively associated with FeNO in atopic children, but not in non-atopic children.19 Our result is the same as above. BHR refers to the degree of airway hyperreactivity in response to a variety of chemical and physical stimuli, which might have little or no effects on FeNO in non-atopic healthy subjects.20 However, little is known about the association between BHR and FeNO levels in healthy children.20 Therefore, further investigations are needed to solve this issue in the general pediatric population.
In the respiratory tract, NO is produced by a wide variety of epithelial cells in the large/peripheral airways and alveoli, by airway and circulatory endothelial cells, and trafficking inflammatory cells.15 NO is a gaseous signaling molecule that is generated by 3 isoenzymes of NO synthase (NOs) that are differentially regulated and expressed in the airways and appear to play different pathophysiologic roles.15 In asthmatic patients, FeNO levels are increased compared to those of healthy subjects. Anti-inflammatory drugs, such as corticosteroids, have been shown to reduce NO concentrations, suggesting that FeNO could be a useful marker of asthma assessment and treatment efficacy.21 Thus, FeNO might be used as a sensitive marker reflecting allergic airway inflammation.
Other markers of airway inflammation include the number of eosinophilis found in induced sputum or bronchoalveolar lavage fluid, and mucosal eosinophilic infiltration seen in fiberoptic bronchial biopsies. Use of these markers requires methods that are too invasive and laborious for use in epidemiologic studies and are unsuitable for routine use, especially in children. Measurements of FeNO are non-invasive and simple to perform in school-age children. This study has shown that FeNO may be significantly associated with peripheral blood eosinophilia in non-atopic, atopic, and all healthy children.
There are, however, several limitations to be considered. First, because we only recruited children from Seoul City, these results are not representative of all Korean children. It should be noted that extrapolation of the results to children living in other areas needs further confirmation. Nevertheless, it can serve as representative data because it shows similar levels compared to Caucasian or northern Chinese children.1,2,14,15 Second, since this was a questionnaire-based study, there are some limitations to interpretation of disease status. However, these limitations was compensated for and overcome by the methacholine challenge test.
The reference values and determinants of FeNO in healthy Korean children established by this study are particularly important for interpreting FeNO test results. The strength of this study is that the group of healthy control children was selected from a large population sample using strict exclusion criteria. Other strengths include a wide participant age range, incorporation of spirometric results and objective markers of atopy, application of current standards for measurement, and a thorough analysis. This was the first study to perform FeNO testing in the general population of Korean school children, rather than in a specific disease population-or hospital-based population, and this study validated the clinical utility of FeNO in the diagnosis of asthma in school children. To eliminate errors, all FeNO measurements were made by the same experienced operator under the supervision of pediatric allergy specialists.
In conclusion, this study established a reference range of FeNO levels in a large group of healthy school-age children. Higher BHR was associated with elevated FeNO levels in all healthy children. FeNO values were independent of age, gender, and BMI in all healthy children. These factors should be considered in future studies when measuring and reporting FeNO, but the mechanisms underlying these relationships require further investigation. We believe that the FeNO reference values and the determinants presented here could be useful for research and clinical practice in Korean children.
Figures and Tables
Figure
Schematic presentation of the recruitment of healthy children. BMI, body mass index; URI, upper respiratory infection.

Table 1
General characteristics of the study subjects

Table 2
Reference FeNO values according to the presence or absence of atopy

| Non-atopic healthy | Atopic healthy | Total healthy | *P value vs atopic | |
|---|---|---|---|---|
| N | 501 | 307 | 808 | |
| Geometric mean (95% CI), ppb | 10.32 (7.95-12.69) | 16.58 (14.03-19.13) | 12.36 (9.85-14.87) | < 0.001 |
Table 3
FeNO values according to gender

Table 4
FeNO values by age
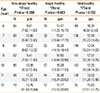
Table 5
Factors affecting FeNO for groups

ACKNOWLEDGMENTS
The authors thank the field workers, elementary school teachers, and other staff who supported data collection, and all the parents and children who participated in this study. This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A092076).
References
1. Malmberg LP, Petäys T, Haahtela T, Laatikainen T, Jousilahti P, Vartiainen E, Mäkelä MJ. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol. 2006; 41:635–642.
2. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005; 115:1130–1136.
3. Kwon JW, Kim BJ, Song Y, Seo JH, Kim TH, Yu J, Kim HB, Lee SY, Kim WK, Kim KW, Ji HM, Kim KE, Kim H, Hong SJ. Changes in the prevalence of childhood asthma in Seoul from 1995 to 2008 and its risk factors. Allergy Asthma Immunol Res. 2011; 3:27–33.
4. Kim WK, Kwon JW, Seo JH, Kim HY, Yu J, Kim BJ, Kim HB, Lee SY, Kim KW, Kang MJ, Shin YJ, Hong SJ. Interaction between IL13 genotype and environmental factors in the risk for allergic rhinitis in Korean children. J Allergy Clin Immunol. 2012; 130:421–426.e5.
5. Lee SY, Kwon JW, Seo JH, Song YH, Kim BJ, Yu J, Park KS, Kim H, Kim EJ, Lee JS, Hong SJ. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012; 158:168–174.
6. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995; 152:1107–1136.
7. Jörres RA, Nowak D, Kirsten D, Grönke L, Magnussen H. A short protocol for methacholine provocation testing adapted to the Rosenthal-Chai dosimeter technique. Chest. 1997; 111:866–869.
8. Kim BJ, Kwon JW, Seo JH, Kim HB, Lee SY, Park KS, Yu J, Kim HC, Leem JH, Sakong J, Kim SY, Lee CG, Kang DM, Ha M, Hong YC, Kwon HJ, Hong SJ. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol. 2011; 107:214–219.e1.
9. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.
10. Lee JY, Seo JH, Kim HY, Jung YH, Kwon JW, Kim BJ, Kim HB, Lee SY, Jang GC, Song DJ, Kim WK, Shim JY, Kim HJ, Shin YJ, Park JW, Cho SH, Lee JS, Hong SJ. Reference values of impulse oscillometry and its utility in the diagnosis of asthma in young Korean children. J Asthma. 2012; 49:811–816.
11. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.
12. Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2008; 133:169–175.
13. Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res. 2009; 10:28.
14. Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003; 21:433–438.
15. Zhang H, Shu L, Cai X, Wang Z, Jiao X, Liu F, Hou P, Wang L, Shan L, Chen N, Shang Y. Gender and age affect the levels of exhaled nitric oxide in healthy children. Exp Ther Med. 2013; 5:1174–1178.
16. Tsang KW, Ip SK, Leung R, Tipoe GL, Chan SL, Shum IH, Ip MS, Yan C, Fung PC, Chan-Yeung M, Lam W. Exhaled nitric oxide: the effects of age, gender and body size. Lung. 2001; 179:83–91.
17. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999; 159:69–73.
18. Kim SH, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS. Reference values and determinants of exhaled nitric oxide in healthy Korean adults. J Asthma. 2010; 47:563–567.
19. Steerenberg PA, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, Opperhuizen A, Brunekreef B, van Amsterdam JG. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003; 58:242–245.
20. Baroffio M, Barisione G, Crimi E, Brusasco V. Noninflammatory mechanisms of airway hyper-responsiveness in bronchial asthma: an overview. Ther Adv Respir Dis. 2009; 3:163–174.
21. Baraldi E, Azzolin NM, Cracco A, Zacchello F. Reference values of exhaled nitric oxide for healthy children 6-15 years old. Pediatr Pulmonol. 1999; 27:54–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download