Abstract
Purpose
The aim of study was to assess the value of recombinants in predicting the degree of symptoms in children with and without anaphylaxis to cow's milk.
Methods
The study included 79 children (70±40 months) referred to the Allergological Unit of the Pediatric Department between the years 2008-2012. Group A was composed of 17 children (78±49.6 months) with anaphylaxis after ingestion of milk. Group B was composed of 62 children (73.1±38.6 months) without a history of anaphylaxis, but with less severe symptoms (gastrointestinal and/or skin symptoms). All patients from Group B had a positive open challenge with cow's milk. All patients underwent an allergic evaluation and blood samples were collected to test for IgE to recombinans of milk (nBos d 4, 5, 8).
Results
A significant difference in nBos d 8 emerged with higher levels in Group A (median [IQR]=2.80 [0.91-16.1]) than B (0.65 [0.24-1.67]; P=0.006), whereas there were no statistically significant differences for nBos d 4 and 5. The recombinants' sum was higher in Group A than B: 8.39 [2.72-41.39] vs 3.04 [1.85-7.31] kUA/L; P=0.044. The recombinant nBos d 8 was superior to the other recombinants in identifying children at risk for anaphylaxis, with an area under the curve of 0.718 (95% CI, 0.57-0.86, P=0.006). Considering a cutoff of 1.8 kUA/L, nBos d 8 had the most favorable sensitivity and specificity ratio (sensitivity=0.65, specificity=0.77) with an odd ratio of 6.02 (95% C.I: 1.89-19.23).
The prevalence of cow's milk allergy (CMA) during infancy ranged from 1.9% in a Finnish study,1 2.16% in statistics from the Isle of Wight,2 2.22% in a study from Denmark,3 2.24% in the Netherlands,4 and up to 4.9% in Norway.5
Cow's milk contains more than 40 proteins and all of them may act as human species antigens. Casein, or nBos d 8, is a major allergen present in milk and the main protein constituent found in cheese, representing 75%-80% of all milk proteins. It is subdivided into a number of families, of which the most important are alpha s1, alpha s2, beta, kappa and gamma-caseins.
These proteins are degraded through proteolytic digestion but are highly resistant to heat; therefore they are little affected by the processes of boiling and pasteurization. This is due to the fact that the main epitopes are linear and not-conformational.6
It has been suggested that the majority of linear IgE epitopes in caseins could contribute to persistent allergies.7 Children allergic to milk who display persistent symptoms have significantly higher levels of specific IgE antibodies to linear epitopes alpha s1 and beta caseins than children who have developed tolerance to milk proteins.8
Beta-lactoglobulin (BLG), also referred to as nBos d 5, is a principal allergen. This protein is highly abundant in whey, accounting for 50% of total protein in the lactoserum fraction and approximately 10% in cow's milk. It has no homologous counterpart in human milk.
Alfa-lactalbumin (ALA), or nBos d 4, is one of the major allergens in cow's milk and represents about 25% of lactoserum (whey) proteins and approximately 5% of cow's milk proteins. It is the main protein in human milk, and, in addition to being the main source of protein for infants, it is a sub-unit of the lactose synthase enzyme and has been shown to be important in the development of the immune system. Epitopes are conformational and have low heat-resistance.
Antibodies to beta-lactoglobulin show 10% cross-reactivity with bovine ALA.
The use of recombinant allergens (native or highly purified) represents a remarkable achievement in allergology for several reasons. They allow to identify the allergenic profile of each patient (Component Resolved Diagnosis, CRD), to define what is the source of the allergen responsible for sensitization and to evaluate the potential danger of sensitization.6
The aim of our study was to assess the value of the recombinant allergens in cow's milk proteins in relation with the degree of clinical symptoms in children with and without anaphylaxis to cow's milk.
The study population included 79 children; 56 males and 23 females (mean [±SD] age: 70 ± 40 months) referred to the Allergological and Respiratory Unit of the Pediatric Department, University of Chieti, Italy, between the years 2008 and 2012.
The sample was divided into 2 Groups: Group A was composed of 17 children (16 males, 1 female) of mean (±SD) age 78±49.6 months who reported a history of anaphylaxis after ingestion of or contact with cow's milk or its derivatives. Group B was composed of 62 children (40 males, 22 females) of mean (±SD) age 73.1±38.6 months without a history of anaphylactic reaction to cow's milk, but who experienced allergic reactions to milk proteins.
Children in Group A were not subjected to oral allergy testing because it would have had a high likelihood of increasing the risk of severe anaphylaxis.9
Children in Group B had a history of allergies to cow's milk but with less severe symptoms. Seventeen children described gastrointestinal symptoms such as abdominal pain, diarrhea or vomiting; 36 patients displayed skin symptoms such as urticaria and atopic dermatitis, and 9 patients had gastrointestinal symptoms associated with skin disease after ingesting cow's milk.
All patients from Group B had a positive open challenge, which according to much literature, is considered the "gold standard" for diagnosing food allergies while minimizing the potential for false positive results.10,11 It was performed using titration steps similar to those used in the double-blind test recommended by the American Academy of Allergy and Immunology.12
All patients underwent an allergological visit and skin prick tests for main food allergens. Subsequently blood samples were collected to test for total and specific IgE to recombinant allergens of cow's milk proteins (alpha-lactalbumin or nBos d 4, beta-lactoglobulin or nBos d 5, and casein or nBos d 8) and for the main food allergens (egg white, egg, tomato, peanut and cod).
Written informed consent was obtained from all parents and oral consent from all children and the study was performed in accordance with Declaration of Helsinki (1964).
Data were analyzed using SPSS (version 17.0 SPSS, Inc, Chicago, III). One sample Kolmogorov-Smirnov test was performed to estimate the distribution of each variable. The Mann-Whitney U test was used for comparisons of continuous parameters (recombinants, the recombinant sum).
Receiver operating characteristic (ROC) analysis was performed to test results in relation to a positive history for anaphylaxis from cow's milk proteins and is presented as the area under the curve with a 95% confidence interval (95% CI). An optimal cutoff point for the recombinants was obtained using the Youden index (maximum [sensitivity + specificity -1]).13
A P value of <0.05 was considered statistically significant.
Group A and B were comparable for age at the time of assessment (Table 1).
By comparing levels of recombinant allergens, a significant difference in nBos d 8 emerged between 2 Groups with higher levels in Group A (median [IQR]= 2.80 [0.91-16.1] kUA/L vs 0.65 [0.24-1.67] kUA/L; P=0.006), whereas there were no statistically significant differences for nBos d 4 and nBos d 5.
The recombinants' sum was higher in Group A than in Group B (8.39 [2.72-41.39] kUA/L vs 3.04 [1.85-7.31] kUA/L; P=0.044) (Table 1).
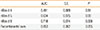
When comparing the different ImmunoCAP tests using ROC analysis, the nBos d 8 ImmunoCAP test was superior to the other recombinants in its ability to differentiate children at risk for cow's milk anaphylaxis, with an area under the curve (AUC) of 0.718 (95% CI, 0.57-0.86, P=0.006) (Figure, Table 2). Neither of the other recombinant allergen immunoCAP tests showed a significant area under the curve (Figure).
A cutoff value of nBos d 8 higher than 1.8 kUA/L, showed the highest Youden index with a sensitivity of 65% and a specificity of 77% (Table 3). Anaphylaxis risk according to cut off value of 1.8 kUA/L was higher in Group A than in Group B (64.7% vs 23.3%) with an odds ratio for the development of cow's milk anaphylaxis of 6.02 [95% C.I.: 1.89-19.23] (Table 4). A better sensibility (77%) was found using a cutoff of 1.02 kUA/l, but with a lower specificity (60%) (Table 3). Anaphylaxis risk according to cut off value of 1.02 kUA/L was higher in Group A than in Group B (76.5% vs 41.7%) with an odd ratio of 4.55 [1.33-15.60] (Table 4).
The present study showed that IgEs to casein (nBos d 8) are superior markers for cow's milk allergy, compared to beta and alpha lactoglobulin with higher levels of nBos d 8 being able to predict anaphylaxis in children allergic to cow's milk proteins.
All children in this study, classified as being allergic, had a history of adverse reactions to cow's milk proteins. Because one group had a history of anaphylaxis, this did not undergo the specific challenging. Meanwhile, the other group was composed of children who did display allergic reactions to cow's milk proteins, but with less severe symptoms.
There is now growing evidence that casein seems to be a major allergen component to be considered when assessing patients with cow's milk allergy.14 D'Urbano et al.15 showed that, patients with positive allergy testing to milk, most frequently had IgE against casein (nBos d 8), compared to other milk allergen components. Children outgrowing their milk allergy have IgE primarily directed at conformational epitopes, whereas children with persistent milk allergy show higher levels of IgE directed to sequential epitopes.16,8 Those children reacting to heated milk have initially higher casein and beta-lactoglobulin IgE levels and are at higher risk for systemic reactions.17
In the study of Garcia-Ara and colleagues on children with cow's milk allergy, it was found that the risk factors for such reactions included very high levels of specific IgE to cow's milk and casein.18 Same research group also studied accidental exposure to milk in children with cow's milk allergy and found that this was a relatively common condition and that 15% of those observed had severe reactions. Risk factors for severe reactions included high levels of IgE against cow's milk and casein in combination with asthma. In line with the above finding, in the present study we found that all patients with anaphylactic reactions to milk proteins had IgE reactivity to casein (nBos d 8) and higher serum levels than allergic children with milder symptoms.
In our study considering a cutoff value of 1.8 KUA/L, nBos d 8 had the most favorable sensitivity and specificity ratio, with a sensitivity of 65% and a specificity of 77%. The specificity was higher than sensibility; these results showed a high positive predictive value of casein in identifying children with risk to develop anaphylaxis. In fact if a patient had a value of nBos d 8 higher than 1.8 KUA/L, the risk of developing cow's milk anaphylaxis was increased to 6 fold.
A better sensitivity (77%) was found using a cutoff of 1.02 kUA/L, but with a lower specificity (60%).
In contrast with our findings, Ott et al.19 found that no single allergen component was a superior discriminator between cow's milk allergy (a positive challenge) and tolerance (a negative challenge).
The combination of all allergen microarray results generated the same or highly similar AUC values as compared to fluorescence enzyme immunoassays (FEIA) testing in cow's milk allergy diagnosis. Comparison of FEIA results with each single microarray component by nonparametric Wilcoxon testing revealed no statistically significant differences between AUC values. The authors therefore claim that there is no possibility to identify single marker allergens that significantly enhance in vitro test performance. Furthermore, the sensitivity of specific IgE to α-casein was lower (26.2%) than that found in our study, while the specificity was higher (97.7%). Sensitivity reached 59% when the 3 recombinant allergens were combined. In fact, the combination of microarrayed cow's milk reduced the possibility of having false negatives.18
In the study from Calvani et al., increased sensitivity to the 3 proteins, measured using the skin prick test, results in the increased likelihood (92.3%) of being allergic and having a positive milk allergy test. In retrospect, applying this criterion earlier on could have saved 42% of all oral tests administered.20 In a subsequent study, the same author reports that children who show positive results for all 3 milk proteins using the skin prick test had a positive challenge result (predictive positive value of 86,7%).21
Hugh et al. have suggested that specific IgE levels for milk >15 kUA/L are the 95% predictive decision points in identifying patients with increased probability of reacting during a specific food challenge.22
In everyday clinical practice, individual recombinant proteins are essential to differentiate between children with genuine sensitivity to cow's milk proteins and to estimate with greater precision the risk of severe reactions after consuming cow's milk.
This study suggested 2 different phenotypes of milk-allergic children, "high-anaphylaxis-risk" and "milder-risk". These types could be differentiated through measuring IgE against casein. Risk assessment of anaphylaxis in children with cow's milk allergy can be improved by using peptide microarray immunoassays to distinguish between those children with less severe symptoms, and those with an increased risk for anaphylaxis symptoms after allergen exposure. Future larger studies are required to confirm our finding and further prove the value of nBos d 8 and/or other recombinant allergens in identifying children at risk of anaphylaxis.
Figures and Tables
 | Fig. 1ROC curve for the recombinant allergens (nBos d 4, nBos d 5, nBos d 8) and the recombinants' sum. |
Table 1
Recombinant levels in children with and without anaphylaxis
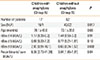
Table 2
Results of ROC analysis for each recombinant and for the recombinant sum
| AUC | S.E. | P | |||
|---|---|---|---|---|---|
| nBos d 4 | 0.491 | 0.089 | 0.91 | ||
| nBos d 5 | 0.634 | 0.075 | 0.09 | ||
| nBos d 8 | 0.718 | 0.074 | 0.006 | ||
| Recombinants' sum | 0.653 | 0.082 | 0.055 | ||
Table 3
Sensitivity and specificity of nBos d 8 for different cutoff levels

| Cut-off for nBos d 8 | Sensitivity | Specificity | Youden index |
|---|---|---|---|
| 0.8 kUA/L | 0.82 | 0.55 | 0.37 |
| 1.02 kUA/L | 0.77 | 0.60 | 0.37 |
| 1.8 kUA/L | 0.65 | 0.77 | 0.41 |
Table 4
Anaphylaxis risk according to different cut-off levels of nBos d 8

References
1. Saarinen KM, Juntunen-Backman K, Järvenpää AL, Kuitunen P, Lope L, Renlund M, Siivola M, Savilahti E. Supplementary feeding in maternity hospitals and the risk of cow's milk allergy: a prospective study of 6209 infants. J Allergy Clin Immunol. 1999; 104:457–461.
2. Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, Dean T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006; 117:1118–1124.
3. Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002; 13:Suppl 15. 23–28.
4. Schrander JJ, van den Bogart JP, Forget PP, Schrander-Stumpel CT, Kuijten RH, Kester AD. Cow's milk protein intolerance in infants under 1 year of age: a prospective epidemiological study. Eur J Pediatr. 1993; 152:640–644.
5. Kvenshagen B, Halvorsen R, Jacobsen M. Adverse reactions to milk in infants. Acta Paediatr. 2008; 97:196–200.
6. Villalta D. CRD nell'allergia alimentare: algoritmi diagnostici (Rassegna). LigandAssay. 2010; 15:18–26.
7. Sicherer SH, Sampson HA. Cow's milk protein-specific IgE concentrations in two age groups of milk-allergic children and in children achieving clinical tolerance. Clin Exp Allergy. 1999; 29:507–512.
8. Vila L, Beyer K, Järvinen KM, Chatchatee P, Bardina L, Sampson HA. Role of conformational and linear epitopes in the achievement of tolerance in cow's milk allergy. Clin Exp Allergy. 2001; 31:1599–1606.
9. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009; 123:S365–S383.
10. Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2009. J Allergy Clin Immunol. 2010; 125:85–97.
11. Sicherer SH. Food allergy: when and how to perform oral food challenges. Pediatr Allergy Immunol. 1999; 10:226–234.
12. Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, Bush RK, Metcalfe DD. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988; 82:986–997.
13. Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006; 163:670–675.
14. Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in pediatric allergology. Pediatr Allergy Immunol. 2011; 22:454–461.
15. D'Urbano LE, Pellegrino K, Artesani MC, Donnanno S, Luciano R, Riccardi C, Tozzi AE, Ravà L, De Benedetti F, Cavagni G. Performance of a component-based allergen-microarray in the diagnosis of cow's milk and hen's egg allergy. Clin Exp Allergy. 2010; 40:1561–1570.
16. Järvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow's milk allergy. J Allergy Clin Immunol. 2002; 110:293–297.
17. Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008; 122:342–347. 347.e1–347.e2.
18. Boyano-Martínez T, García-Ara C, Pedrosa M, Díaz-Pena JM, Quirce S. Accidental allergic reactions in children allergic to cow's milk proteins. J Allergy Clin Immunol. 2009; 123:883–888.
19. Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, Niggemann B, Beyer K. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008; 63:1521–1528.
20. Calvani M, Alessandri C, Frediani T, Lucarelli S, Miceli Sopo S, Panetta V, Zappalã D, Zicari AM. Correlation between skin prick test using commercial extract of cow's milk protein and fresh milk and food challenges. Pediatr Allergy Immunol. 2007; 18:583–588.
21. Calvani M, Berti I, Fiocchi A, Galli E, Giorgio V, Martelli A, Miceli Sopo S, Panetta V. Oral food challenge: safety, adherence to guidelines and predictive value of skin prick testing. Pediatr Allergy Immunol. 2012; 23:755–761.
22. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–896.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download