Abstract
Purpose
Specific oral immunotherapy (SOIT) using interferon-γ (IFN-γ) has been successful as a food allergy treatment. Interleukin-10 (IL-10)-producing regulatory B cells (Br1s) play a role in immune tolerance to food allergens. In addition, IFN-γ shows tolerogenic effects on allergen-induced Br1 responses.
Methods
Eleven patients that were allergic to cow's milk and 12 milk-tolerant subjects were selected by double-blind placebo-controlled food challenge (DBPCFC) and clinical characteristics. The immunomodulatory effects of IFN-γ on allergen-specific Br1 responses were evaluated in 6 milk allergy patients and 8 milk-tolerant subjects. Peripheral blood mononuclear cells (PBMCs) from subjects were stimulated with casein and/or IFN-γ and analyzed by flow cytometry.
Results
IFN-γ had no effect on total cell counts or the proportion of Br1 cells in PBMCs. IFN-γ stimulation did not change total Br1 cell counts or the percentage of Br1s among CD5(+) B cells in the milk allergy or the milk-tolerant groups. In the milk allergy group, Br1 counts were not different between the control and the casein stimulation but significantly increased in the IFN-γ + casein stimulated cells, and the Br1 fractions were decreased after casein stimulation and recovered in the addition of IFN-γ for stimulation. In the milk-tolerant group, Br1 counts increased in the casein stimulated cells and in the IFN-γ + casein stimulated cells, but the increase was significantly less when IFN-γ was added, and the Br1 fractions were increased after casein stimulation and IFN-γ + casein stimulation, that was not significant when IFN-γ was added.
Conclusions
IFN-γ-induced allergen-specific Br1 responses in the PBMCs of milk allergy patients play a role in milk allergen-specific tolerance induction in vitro. Further investigations into the molecular immunological mechanisms underlying the induction of allergen-specific Br1 responses are needed.
Food allergy is an abnormal response triggered by the immune system. Food allergy is classified as an immunoglobulin E (IgE)-mediated, non-IgE mediated, or a mixed-type allergy. Late eczematous skin reactions are typical and diagnostic symptoms of non-IgE mediated and mixed-type allergies.1-3 The standard treatment for food allergy is elimination of the incriminated food from the diet.4 Tolerance induction is an important concern regarding the treatment of food allergies. Specific oral immunotherapy (SOIT) is achieved by oral exposure to increasing doses of a food allergen and is a valid treatment option for persistent food allergy. Oral immunotherapy (OIT) as a treatment for food allergies has been discussed since 1996.5 The induction of specific food allergen oral tolerance has had limited success.6,7 However, specific oral tolerance induction to food allergens using interferon-γ (IFN-γ) was successful in milk allergy patients with late eczematous reactions.8 IFN-γ is a Type 1 helper T cell (Th1) cytokine and plays central roles in the Th1/Type 2 helper T cell (Th2) balance.9 SOIT with IFN-γ may induce tolerance induction in IgE-mediated10,11 and non-IgE-mediated food allergies.11 IFN-γ appears to play an important role in food allergen tolerance induction, especially in non-IgE-mediated food allergy.
Immune tolerance comprises complex immunological mechanisms involving both cellular and humoral immune components, including cytokines. CD4+Foxp3+ regulatory T cells play a central role in immune tolerance.12 CD5(+) B cells, also known as regulatory B cells, are a subset of B cells that respond during late eczematous reactions from cow's milk allergy by producing interleukin-10 (IL-10).13-16 IL-10-producing regulatory B cells (Br1s), a subset of CD5(+) regulatory B cells, prevent and reverse allergic airway inflammation via Foxp3(+) T regulatory cells in a murine model.17 Br1s were implicated in food allergen tolerance in our previous reports.15 Allergen-specific Br1 responses are induced by IFN-γ in vivo and are associated with tolerance induction for non-IgE-mediated food allergy.18 Based on these data, we investigated allergen-specific Br1 responses in milk allergy patients and milk-tolerant subjects, focusing on the role of IFN-γ as a mechanism of tolerance induction to allergens that cause late eczematous skin reactions.
Subjects between 10 and 28 years of age who suffered from a repeated history of late eczematous reactions or exacerbations of atopic dermatitis (AD) and who were treated in the Department of Pediatrics, Eulji University Hospital, and the Seoul Allergy Clinic were screened for the current study. No other allergic symptoms, such as gastrointestinal or respiratory manifestations, were present. All prospective subjects received blood tests and skin prick tests as described below and fulfilled the criteria of Hanifin and Rajka.19 Signed consent forms were obtained from either the patient or the parent. The study was approved by the Institutional Review Board (IRB) of Eulji University Hospital, Daejeon, Korea.
Double-blind placebo-controlled food challenge (DBPCFC) tests were conducted for milk as described below. The diagnostic criteria for late eczematous food allergy reactions are well described in previous reports and include the following: 1) late eczematous reactions including exacerbation of AD by DBPCFC, 2) exacerbation onset four hours after the food challenge, and 3) negative skin prick test reactions and food-specific IgE levels for milk.14 Eleven subjects with milk allergies according to the late eczematous reaction diagnostic criteria for milk allergy were selected as the milk allergy group, and 12 subjects who did not react to milk were selected as the milk-tolerant group (Table). The in vitro effects of IFN-γ on Br1 responses and allergen-specific Br1 responses were evaluated experimentally in 6 individuals from the milk allergy group and 8 individuals from the milk-tolerant group.
Subjects received blood tests and skin prick tests. Blood tests included complete blood counts with a differential count for the eosinophil fraction, total serum IgE levels, and blood-specific IgE for milk (Uni-CAP; Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden). Serum food-specific IgE levels less than 0.35 kUA/L were classified as undetectable. Skin prick tests for milk were conducted on the patient's left forearm using commercial allergen extracts (Bencard, Brentford, UK). Histamine hydrochloride (1 mg/mL) (Bencard) was used as a positive control and physiological saline was used as a negative control. Reactions were read after 15 minutes and classified as negative (0: no reaction, +: reaction greater than the control reaction, but smaller than half that caused by histamine) or positive (++: half the level, +++: equal to, and ++++: twice the level caused by histamine).
To diagnose milk allergies, the DBPCFC test was conducted according to the diagnostic methods used in previous reports.14 Briefly, an elimination diet for all possible foods suspected of provoking allergic AD was used. Suspected foods were eliminated for at least two weeks.20,21 The DBPCFC test was conducted just after administration of the elimination diet. The DBPCFC test was performed using a mixed cereal flour vehicle consisting of brown rice flour, glutinous rice flour, and barley flour. Two challenges were performed five days apart; one was the placebo only, while the other contained the suspected food antigen in the vehicle. Randomization and preparation of the challenges were performed by dieticians in the clinical research unit. All reactions were scored as to type, time of onset, severity, and duration.
Diagnostic criteria for milk allergy included: 1) a late eczematous reaction characterized by typical erythematous eruption of eczematous skin lesions22 and 2) onset time later than 6 hours after food challenge.23
The signs and symptoms of milk allergy appeared as newly developed symptoms or exacerbations of AD. The clinical severity was evaluated using the SCORing Atopic Dermatitis (SCORAD) index, a system used worldwide to assess the severity of atopic eczema.24 Diagnosis of milk allergy was made if the clinical severity score increased by more than 20% in subjects demonstrating a basal score greater than 50 points, or if the score increased by over 10 points in those with a basal score less than or equal to 50 points. New skin lesions and itching were regarded as positive reactions, which were required for the diagnosis of milk allergy in response to the milk challenge.
Peripheral blood mononuclear cells were isolated from venous blood by density-gradient separation using Ficoll-Hypaque (Biomedicals, Aurora, OH, USA) and were then resuspended at 1×106 cells/mL in α-minimum essential medium (alpha-MEM; Irvine Scientific, Santa Ana, CA). Peripheral blood mononuclear cells were stimulated with IFN-γ only (IFN), casein only (Casein), or IFN-γ with casein (IFN+Casein). A 1×106 cells/mL aliquot that was cultured without casein was used as an unstimulated control (None). PBMCs were cultured in 24-well plates at a concentration of 1×106 cells/mL with or without a 50 µg/mL mixture of purified alpha-, beta-, and kappa-caseins (Sigma-Aldrich, St. Louis, MO, USA). The final concentrations of casein (1 mg/mL) and IFN-γ (10 µg/mL) were the same as those used in a previous study.14
After PBMCs were cultured with or without casein or IFN-γ for 18 hours, cells were stained with specific monoclonal antibodies as described below. The allophycocyanin (APC)-labeled anti-CD19 (eBioscience, San Diego, CA, USA) and phycoerythrin Cy7 (PE.Cy7)-labeled anti-CD5 (eBioscience, San Diego, CA, USA) were used for B cell subset analysis. The cells were prepared at a concentration of 1×106 in 100 mL of FACS staining buffer (eBioscience, San Diego, CA, USA) in an Eppendorf tube. Monoclonal antibodies were added at a concentration of 1 mg/mL according to the manufacturer's instructions. After incubation for one hour in a dark room and three washes with FACS staining buffer, cells were resuspended in 500 mL of FACS staining buffer just before flow cytometric analysis. After finishing cell surface staining, permeabilization/fixation of the cells was performed using a permeabilization/fixation kit (eBioscience, San Diego, CA, USA). Intracellular IL-10 was stained using a phycoerythrin-labeled anti-IL-10 rat IgG1 monoclonal antibody (eBioscience, San Diego, CA, USA). Stained cells were acquired using a FACSCaliber device (BD, Biosciences, Milpitas, CA, USA), and the data were analyzed using CellQuest software (BD, Biosciences).
Statistical analyses were performed using the Wilcoxon signed rank sum test. Specifically, we compared changes in the cellular fraction caused by allergen stimulation in the milk allergy and milk-tolerant groups. SigmaPlot Version 11 (Systat Software Inc., San Jose, CA, USA) was used for all statistical analyses. P<0.05 was considered to be statistically significant for all analyses.
Milk allergy subjects developed late eczematous lesions after DBPCFC for milk. All milk-allergic and milk-tolerant subjects showed negative skin prick test results for milk and insignificant/undetectable milk-specific IgE levels (Table).
CD19(+)CD5(+) B cells were isolated following expression of CD5 and CD19 in cultured PBMCs. B cells were subgated according to intracellular IL-10 staining (Fig. 1A). PBMCs from milk allergy patients (n=6) and milk-tolerant subjects (n=8) were stimulated with IFN-γ, and the effects of IFN-γ on Br1 responses
were examined. IFN-γ had no effect on total cell counts or on the proportion of Br1s in PBMCs (Fig. 1B). Br1 cells were not significantly different following IFN-γ stimulation in the milk allergy group (from 91.1±46.1 to 129.0±175.0; P=0.297) or the milk-tolerant group (from 43.4±10.8 to 92.6±105.3; P=0.156) (Fig. 1C). In addition, the percentage of Br1s among CD5(+) B cells was not significantly different following IFN-γ stimulation in the milk allergy group (from 24.4±9.9% to 28.8±18.3%; P=0.291) or the milk-tolerant group (from 9.4±4.6% to 18.4±12.1%; P=0.072) (Fig. 1D).
The effects of IFN-γ on allergen-specific Br1 responses in the milk allergy (n=6) and milk-tolerant subjects (n=8) were evaluated in vitro by stimulating PBMCs with casein only (Casein), IFN-γ with casein (IFN+Casein), or without any stimulant as a control (None) (Fig. 2A). In the milk allergy group, Br1 cell numbers were not significantly different between the control (91.1±45.1) and casein stimulation (82.4±38.3; None vs. Casein, P=0.271) groups, but increased to 116.9±48.4 with IFN-γ+casein stimulation (None vs. IFN+Casein, P=0.038; Ag vs. IFN+Casein, P=0.006). In the milk-tolerant group, Br1 cells increased from 43.4±10.8 (None) to 279.6±276.9 with casein stimulation (Casein) (None vs. Casein, P=0.043), and to 116.9±48.4 with IFN-γ and casein stimulation (IFN+Casein) (None vs. IFN+Casein, P=0.028). The increase was significantly less when IFN-γ was added (Casein vs. IFN+Casein, P=0.010) (Fig. 2B). Thus, allergen-specific Br1 responses were induced by IFN-γ in milk allergy patients; however, these responses were suppressed by IFN-γ in the milk-tolerant subjects.
In the milk allergy group, the Br1 fractions decreased from 24.4±9.8% (None) to 15.0±10.0% (Casein) (None vs. Casein, P=0.002) after casein stimulation and recovered to 22.6±8.5% when casein stimulation occurred in the presence of IFN-γ (IFN+Casein) (None vs. IFN+Casein, P=0.208; Casein vs. IFN+Casein, P=0.006). In the milk-tolerant group, Br1 fractions increased from 9.4±4.6% (None) to 17.1±6.6% (Casein) after casein stimulation, and to 15.7±8.7% when casein stimulation occurred in the presence of IFN-γ (IFN+Casein) (None vs. Casein, P=0.014; None vs. IFN+Casein, P=0.066). This increase, however, was not significantly different when IFN-γ was added (Casein vs. IFN+Casein, P=0.295) (Fig. 2C). Allergen-specific Br1 responses, which were measured as the proportion of Br1s among CD5(+) B cells, were induced by IFN-γ in milk allergy patients and were not significantly different following the addition of IFN-γ in milk-tolerant subjects.
The in vitro effects of IFN-γ on allergen-specific Br1 responses were investigated in the present study as a possible immunological mechanism underlying tolerance induction to non-IgE-mediated food allergies. SOIT without IFN-γ for the treatment of IgE-mediated food allergies has been attempted with limited success in other studies.6,7 IFN-γ has been introduced successfully as an immunomodulatory drug for the treatment of IgE-mediated and non-IgE-mediated food allergies associated with AD.6,10 In addition, the clinical and therapeutic characteristics of SOIT using IFN-γ are well described.10,11 Allergen-specific Br1 responses were induced by the stimulation of PBMCs from non-IgE-mediated milk allergy patients with both allergen and IFN-γ in vitro (Fig. 2).
Regulatory B cells are involved in the pathogenesis of delayed-type hypersensitivity via early T cell recruitment.25 Regulatory B cells also mediate airway hyperreactivity in non-atopic asthma.26 However, regulatory B cells play a negative regulatory role by producing IL-10 and inducing regulatory T cells.15 Moreover, allergen-specific Br1 responses are related to the tolerance of food allergens that cause late eczematous reactions.15,17 A similar effect is seen by the induction of IL-10-producing CD4(+)CD25(+) T cells during grass pollen immunotherapy.27 These results support the hypothesis that IL-10-producing CD19(+)CD5(+) regulatory B cells (Br1s), in addition to IL-10-producing CD4(+)CD25(+) regulatory T cells (Tr1s), play an important role in food allergen tolerance. In this study, Br1 cell counts increased during casein stimulation in the milk-tolerant group, but not in the milk allergy group (Fig. 2). This result suggests that Br1 cells are involved in the pathogenesis of tolerance induction to food allergens.
Br1 responses were not induced by IFN-γ therapy alone without allergen stimulation (Fig. 1), but were induced when IFN-γ was simultaneously administered with allergen (Fig. 2). These in vitro results indicate that IFN-γ induces allergen-specific Br1 responses in an allergen-dependent manner and modulates allergen-specific immune responses. In the case of CD8(+) T cells, the antigen-dependent T cell receptor (TCR)-mediated pathway is important for peripheral tolerance induction.28 However, the TCR-mediated immunomodulatory effects of IFN-γ on the induction of allergen-specific Br1 responses in milk allergy patients requires further investigation. Morphologically, IFN-γ transforms CD5+ B cells into macrophage-like cells, which play a role in cellular immunological defenses. However, IL-4 converts CD5+ B cells into lymphocyte-like cells, which play a role in human immunology.29 It is unclear whether IFN-γ functionally affected Br1s, as IFN-γ did not quantitatively change Br1 responses in PBMCs (Fig. 1).
Differential immunomodulatory effects of IFN-γ on allergen-specific Br1 responses were observed between milk allergy and milk-tolerant subjects (Fig. 2). IFN-γ may invoke allergen-specific tolerogenic effects in part by inducing immunosuppressive allergen-specific Br1 responses in milk allergy patients. IFN-γ also appeared to prevent excessive immunosuppression by decreasing allergen-specific Br1 responses in milk-tolerant subjects.
IFN-γ production in response to allergen stimulation was relatively
deficient in milk allergy patients as compared with milk-tolerant subjects. In a previous study, allergen-specific IFN-γ production in PBMCs was abolished by oral challenge in patients
who developed AD due to a cow milk allergy.30 During oral tolerance induction, IFN-γ production by T cells was also reported.31 Further, IFN-γ generation was defective in patients with cow's milk allergies.32
These data support the hypothesis that IFN-γ supplementation is complementary and suggests that the IFN-γ defect could be treated to achieve normal tolerant immune responses to milk. The involvement and/or requirement of IFN-γ in immune tolerance was reported by several groups. Tolerance induction to peanut allergens by herbal formula-2 was associated with the upregulation of IFN-γ33 by IFN-γ-producing CD8(+) T cells.34 However, it was unclear whether IFN-γ upregulation was secondary to tolerance acquisition or was the primary cause of the successful induction of food allergen tolerance. In our experiments, IFN-γ was related to allergen-specific Br1 responses, resulting in an inevitable correlation with immune tolerance induction to the food allergen.
IL-10-producing T regulatory cells (Tr1s) are induced by sublingual immunotherapy35 and Th3-type TGF-β-secreting regulatory cells,36 which upregulate TGF-β secretion.37 IL-10 and TGF-β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and during specific immunotherapy after tolerance to a specific antigen.38 TGF-β and IL-10 are potent immunosuppressive cytokines.39 Moreover, the collective data support inference that IL-10 and TGF-β are the major cytokines involved in immune tolerance, and that these cytokines are therapeutically beneficial. However, the persistent effects of these cytokines are worrisome. Specifically, they can lead to serious complications, such as infection and cancer, due to immune surveillance system dysfunction via non-specific immunosuppression. IFN-γ has several advantages over TGF-β and IL-10 for inducing tolerance. For example, IFN-γ is generally a proinflammatory cytokine, and there are no concerns regarding infection or cancer development, as it prevents or improves immune functions. Further, IFN-γ has anti-allergic effects and induces tolerance to specific allergens in allergic diseases and diseases in which allergic mechanisms are involved. Lastly, IFN-γ selectively mediates immunological functions according to the type of immune response, such as IFN-γ-induced allergen-specific Br1 responses in milk allergy patients and reduced allergen-specific Br1 responses in milk-tolerant subjects.
In conclusion, IFN-γ induced allergen-specific Br1 responses and facilitated a switch from allergic to tolerant status in an allergen-specific manner. In addition, IL-10-producing CD5(+) regulatory B cells were involved in immune tolerance to food allergens. IFN-γ appears to be a relevant cytokine in the acquisition of tolerance to foods that should be tolerated by normal immunological processes. Further studies on the molecular and immunological mechanisms of IFN-γ in tolerance induction are warranted.
Figures and Tables
Fig. 1
Non-specific effects of interferon-γ (IFN-γ) on interleukin-10 (IL-10)-producing regulatory B cell (Br1) responses in peripheral blood mononuclear cells (PBMCs). (A) Gating of CD19(+)CD5(+) B cells and subgating of IL-10-producing regulatory B cells. (B) Representative plot of IL-10-producing regulatory B cell (Br1) responses induced by IFN-γ simulation of PBMCs from milk allergy and milk-tolerant subjects. Numbers indicate the percentage of cells. (C) Changes in Br1 cell numbers caused by IFN-γ in milk allergy patients and milk-tolerant subjects. (D) Changes in the proportion of Br1 cells to CD5(+) B cells as a result of IFN-γ treatment. Positive, milk allergy patient (DBPCFC positive); Negative, milk-tolerant subject (DBPCFC negative); None, unstimulated PBMCs.
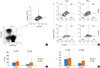
Fig. 2
Immunomodulatory effects of interferon-γ (IFN-γ) on allergen-specific interleukin-10 (IL-10)-producing regulatory B cell (Br1) responses in peripheral blood mononuclear cells (PBMCs). (A) Representative plot of allergen-specific Br1 responses of cells from milk allergy patients and milk-tolerant subjects induced by stimulation with casein and/or IFN-γ. Numbers indicate the percentage of cells. (B) Allergen-specific changes in Br1 cell numbers in milk allergy patients and milk-tolerant subjects. (C) Changes in the proportion of Br1 cells to CD5(+) B cells as a result of IFN-γ treatment. Positive, milk allergy patient (DBPCFC positive); Negative, milk-tolerant subject (DBPCFC negative); None, unstimulated PBMCs; Casein, PBMCs stimulated with casein; IFN+Casein, PBMCs stimulated with IFN-γ and casein simultaneously.

Table
Subject profiles

ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0021633).
References
1. Werfel T, Ballmer-Weber B, Eigenmann PA, Niggemann B, Rancé F, Turjanmaa K, Worm M. Eczematous reactions to food in atopic eczema: position paper of the EAACI and GA2LEN. Allergy. 2007. 62:723–728.
2. Wang J, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Allergy Asthma Immunol Res. 2009. 1:19–29.
3. Cianferoni A, Spergel JM. Food allergy: review, classification and diagnosis. Allergol Int. 2009. 58:457–466.
4. Fogg MI, Spergel JM. Management of food allergies. Expert Opin Pharmacother. 2003. 4:1025–1037.
5. Wüthrich B. Oral desensitization with cow's milk in cow's milk allergy. Pro! Monogr Allergy. 1996. 32:236–240.
6. Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005. 60:1320–1322.
7. Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007. 62:1261–1269.
8. Noh G, Lee SS. Marone G, editor. Effects of IFN-γ on milk-specific oral tolerance induction for milk allergy in atopic dermatitis: food-specific oral tolerance induction using IFN-γ as adjuvant. Clinical immunology and allergy in medicine. 2003. Naples, Italy: JGC Editions;475–496.
9. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998. 19:359–361.
10. Noh G, Lee SS. A pilot study of interferon-gamma-induced specific oral tolerance induction (ISOTI) for immunoglobulin E-mediated anaphylactic food allergy. J Interferon Cytokine Res. 2009. 29:667–675.
11. Lee JH, Noh G, Noh J, Lee S, Choi WS, Kim HS, Lee K, Choi S, Jin H, Cho S, Lee S. Clinical characteristics of oral tolerance induction of IgE-mediated and non-IgE-mediated food allergy using interferon gamma. Allergy Asthma Proc. 2010. 31:e39–e47.
12. Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009. 123:43–52.
13. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006. 176:705–710.
14. Lee JH, Noh J, Noh G, Kim HS, Mun SH, Choi WS, Cho S, Lee S. Allergen-specific B cell subset responses in cow's milk allergy of late eczematous reactions in atopic dermatitis. Cell Immunol. 2010. 262:44–51.
15. Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, Cho S, Lee SS. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in Cow Milk Allergy. Cell Immunol. 2010. 264:143–149.
16. Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011. 3:168–177.
17. Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010. 125:1114–1124.
18. Noh J, Noh G, Lee SJ, Lee JH, Kim A, Kim HS, Choi WS. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell Immunol. 2012. 273:140–149.
19. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 59:Suppl 92. 44–47.
20. Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, Bush RK, Metcalfe DD. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988. 82:986–997.
21. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009. 123:S365–S383.
22. Hauk PJ. The role of food allergy in atopic dermatitis. Curr Allergy Asthma Rep. 2008. 8:188–194.
23. Werfel T, Breuer K. Role of food allergy in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004. 4:379–385.
24. Sprikkelman AB, Tupker RA, Burgerhof H, Schouten JP, Brand PL, Heymans HS, van Aalderen WM. Severity scoring of atopic dermatitis: a comparison of three scoring systems. Allergy. 1997. 52:944–949.
25. Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003. 171:6225–6235.
26. Kawikova I, Paliwal V, Szczepanik M, Itakura A, Fukui M, Campos RA, Geba GP, Homer RJ, Iliopoulou BP, Pober JS, Tsuji RF, Askenase PW. Airway hyper-reactivity mediated by B-1 cell immunoglobulin M antibody generating complement C5a at 1 day post-immunization in a murine hapten model of non-atopic asthma. Immunology. 2004. 113:234–245.
27. Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003. 111:1255–1261.
28. Vacchio MS, Hodes RJ. CD28 costimulation is required for in vivo induction of peripheral tolerance in CD8 T cells. J Exp Med. 2003. 197:19–26.
29. Koide N, Sugiyama T, Mori I, Mu MM, Hamano T, Yoshida T, Yokochi T. Change of mouse CD5(+) B1 cells to a macrophage-like morphology induced by gamma interferon and inhibited by interleukin-4. Clin Diagn Lab Immunol. 2002. 9:1169–1174.
30. Sütas Y, Hurme M, Isolauri E. Oral cow milk challenge abolishes antigen-specific interferon-gamma production in the peripheral blood of children with atopic dermatitis and cow milk allergy. Clin Exp Allergy. 1997. 27:277–283.
31. Lee HO, Miller SD, Hurst SD, Tan LJ, Cooper CJ, Barrett TA. Interferon gamma induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology. 2000. 119:129–138.
32. Suomalainen H, Soppi E, Laine S, Isolauri E. Immunologic disturbances in cow's milk allergy, 2: Evidence for defective interferon-gamma generation. Pediatr Allergy Immunol. 1993. 4:203–207.
33. Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007. 37:846–855.
34. Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009. 123:443–451.
35. Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007. 120:707–713.
36. Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001. 3:947–954.
37. Sato MN, Fusaro AE, Victor JR, Oliveira CR, Futata ET, Maciel M, Carvalho AF, Duarte AJ. Oral tolerance induction in dermatophagoides pteronyssinus-sensitized mice induces inhibition of IgE response and upregulation of TGF-beta secretion. J Interferon Cytokine Res. 2001. 21:827–833.
38. Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003. 33:1205–1214.
39. Kehrl JH, Taylor A, Kim SJ, Fauci AS. Transforming growth factor-beta is a potent negative regulator of human lymphocytes. Ann N Y Acad Sci. 1991. 628:345–353.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download