Abstract
Purpose
The mast cell plays a pivotal role in the human immune response. Crosslinking of 2 IgE molecules bound to the high affinity IgE receptor (FcεRI) on the surface of the mast cell results in mast cell degranulation and the release of several proinflammatory mediators. Patients with type-I allergy have increased levels of IgE in the blood compared to healthy individuals.
Methods
In a 6-week culture system of stem cells to human mast cells we investigated the effect of the concentration of IgE. The mast cells were cultured with different concentrations of IgE for the last 10 days of the maturation period. It was observed how the IgE concentration affects the histamine release, FcεRI density on the mast cell surface and the concentration of other mediators.
Results
A clear correlation between IgE concentration in culture medium and the release of histamine upon activation was observed. It showed a bell-shaped dose response curve, with maximal response around an IgE-concentration of 250 ng/mL. Furthermore, the sensitivity of the mast cells and surface density of FcεRI on mast cell surface was also influenced by the IgE concentration in the culture medium.
Conclusions
IgE in the culture medium during the last 10 days of mast cell maturation influences the release of the preformed mediator histamine after mast cell activation and the density of FcεRI on the mast cell surface. The release of the de novo synthetized mediator prostaglandin D2 and the expression of chymase and tryptase are not influenced by IgE in culture medium.
The mast cell is a myeloid cell which characteristically leaves the bone marrow as an immature progenitor stem cell and subsequently homes in the various tissues of the respiratory and gastrointestinal tracts, in the skin and in connective tissues along blood vessels where it undergoes its final maturation and resides. It is therefore interesting if the IgE concentration surrounding the mast cells during their final maturation influences the mast cells ability to produce and release mediators like histamine and prostaglandin D2 (PGD2). The cytoplasm of the resting mast cell is filled with large granules containing histamine, prostaglandins, and other proinflammatory mediators. The activation and degranulation of mast cells make a major contribution to inflammation. Thus, mast cells have a pivotal role as an effector cell in type-I allergic disorders for instance asthma and autoimmune diseases like rheumatoid arthritis. The mast cell express on its surface FcεRI receptor and an array of other receptors like G protein-coupled receptors and Toll-like receptors, all involved in initiation and amplification of the allergic response upon activation.1-5
The aggregation of FcεRI can be induced by stimulation of IgE-sensitized mast cells with specific allergen or anti-IgE. In response to allergen exposure the mast cell releases preformed mediators like histamine and tryptase along with newly synthesized arachidonic acid metabolites like PGD2 and leukotrienes and other cytokines and chemokines.2 The release of these immunoreactive substances result in increased vascular permeability, contraction of smooth muscles surrounding the airways (bronchoconstriction) and enhances chemokine production.1
IgE antibodies against a wide variety of different antigens are normally present in small amounts in the blood stream. In humans, both total and specific IgE levels usually correlate positively with the presence of type-I allergy symptoms.6,7 Although the positive correlation of both total and specific IgE with type-I allergy, the level of total and specific IgE cannot, by itself, be a useful tool for diagnosis of allergy.6,7 The elevated levels of IgE found in patients with type-1 asthma and allergy correlate with higher FcεRI expression on the cell surface. The receptor stabilization by IgE at the plasma membrane delays breakdown, and due to the continued basal receptor synthesis, the number of IgE receptors on the surface increases.8
Both studies of humans and animals with different parasite infections have shown that these infections often result in an increased synthesis of nonspecific polyclonal IgE.8,9 Other studies of populations in rural areas in Brazil and Ecuador revealed a strong inverse correlation between high IgE-levels due to parasite infections and reactivity to common allergens.10,11 It is hypothesized that the increased levels of parasite IgE competes for the same FcεRI receptors as allergen-specific IgE, thereby blocking the allergen-induced degranulation of mast cells.10 A previous study of Brazilian children showed that an infection with Trichuris trichiura early in life resulted in a lower prevalence of allergen skin test reactivity later in childhood.12 The negative relation between parasite infection and allergy-related symptoms was supported by experiments with anti-parasite treatment. A Gabonese study showed that repeated treatments of parasite infection resulted in a significant increase in the rate of developing skin-test sensitivity to house dust mites.13 All these results points to the fact that the concentration of non-specific IgE has an influence on the mast cell activation.
In this study mast cell were cultured with different concentrations of IgE corresponding to the physiological range. The influence of IgE levels on activation of the mature mast cells was examined.
Ficoll-paque solution was purchased from Amersham Pharmia Biotech (Uppsala, Sweden). MACS-columns and buffer and AC133 cell isolation kits were purchased from Miltenyi Biotech (Bergisch-Gladbach, Germany). StemSpan medium was bought from Stem Cell Technologies (Vancouver, Canada). Recombinant human IL-3, SCF and IL-6 were purchased from R&D Systems (Abingdon, UK). Fetal Calf Serum and penicillin/streptomycin was purchased from GIBCO BRL (Grand Island, NY, USA). Chymase and tryptase kits and QIFIKIT® were purchased from Dako (Glostrup, Denmark).
Human peripheral blood was obtained from the local blood bank from voluntary blood donors. The blood was diluted 3 times and overlaid a Ficoll-paque gradient. The gradient was centrifuged for 30 min, 22℃ and 1,500 rpm. The mononuclear cells were isolated and washed twice with PBS and once with MACS buffer by centrifugation for 5 min, 5℃ and 1,500 rpm. Human CD133+ cells were isolated using AC133 micro cell isolation kit following the manufacturer's instructions. Briefly, unspecific binding of the antibodies on the cell surface was blocked by FcR blocking reagent, before the cells were incubated with AC133 microBeads for 30 min at 4℃. After incubation with beads, the cells were washed with MACS buffer before separation on a magnetic LS+ separation column placed in a magnetic field, retaining the CD133+ cells. The column was washed 4 times before it was removed from the magnetic field, and the CD133+ cells were eluted with MACS buffer. Finally, the cells were counted in Trypan blue to determine viability, and transferred to culture medium.14
Purified CD133+ cells were cultured for seven weeks giving mature human mast cells. The cells were cultured in StemSpan serum-free medium containing 50 ng/mL rh-IL-5, 100 ng/mL rh-SCF and 100 µg/mL penicillin/streptomycin. Week 1-3 the medium was supplemented with 1 ng/mL rh-IL-3 to induce proliferation, from week 6 10% FCS was added to promote the final differentiation to mature mast cells. Once a week the cells were counted, metachromatic stained with Alcian blue, and passed on to fresh culture medium.14,15
The cells were cultured as described above. The last ten days of the 7 week culture period the cell culture was split into separate cultures supplemented with the following concentrations of myeloma IgE: 0, 50, 100, 250, 500 ng/mL, 1, 2, 5 µg/mL. After 10 days incubation with IgE the cells were washed and resuspended in Pipes buffer (10 mM pipes, 150 nM Na-acetate, 5 nM K-acetate, 0.6 mM CaCl2, 1.1 mM MgCl2, 1 mg/mL glucose, 0.3 mg/mL HAS, 15 IE/mL heparin added 100 ng/mL rhSCF and 50 ng/mL rhIL-6, pH 7.4). The cells were activated by adding 5 µg/mL anti-IgE at 37℃. After 30 min the activation reaction was stopped with cold Pipes Stam buffer. The cells were centrifuged for 5 min, 4℃ and 1,500 rpm. For histamine release and PGD2 secretion 20,000 and 10,000 cells were used, respectively.
For analysis of histamine release, the supernatant and pellet were frozen separately at -20℃ before analysis. The histamine release was measured using a fluorescence based method.16 The samples were boiled for 10 min before centrifugation for 10 min, 4℃ and 14,000 rpm. The samples were loaded to microtiter plate containing microfiber pads that binds histamine.
The influence of IgE on the sensitivity to anti-IgE concentration was determined by titration with anti-IgE with concentrations from 5,000 ng/mL to zero. By sigmoid fitting of the data the EC50 (effective concentration) values are calculated. The EC50 value refers to the concentration of anti-IgE which induces a response halfway between the baseline and maximum release of histamine.17
For analysis of PGD2 a Prostaglandin D2-MOX EIA kit from Caymann Chemicals was used according to manufacturer's instructions.
The density of FcεRI on mast cell surface was measured using QIFIKIT® from Dako. The cells were cultured for 10 days with the following IgE concentrations: 0, 100, 250, 1,000, and 5,000 ng/mL. Afterwards the cells were analyzed by flow cytometry according to the manufacturer's instructions.
Immunostaining of human mast cells was performed using a kit from Dako. 75,000 cells were spun down onto a glass slide. The cells were left to air-dry and afterwards fixated in a 1:1 methanol: ethanol solution. The cytospins were incubated with primary antibody for 24 h at 4℃ in a humidified chamber, after washing with tris buffered saline (TBS), the secondary antibody was added and incubated for 30 min. Afterwards the cells were stained by incubating with alkaline phosphatase anti-alkaline phosphatase (APAAP method) for 30 min at room temperature.
The results are given as means±standard deviations. After a positive normality-test, the statistical significance was analyses by using paired or unpaired T-test. When data was not normally distributed Mann-Whitney test was used. SigmaPlot for Windows Version 11.0 (Systat Software, San Jose, CA, USA) was used for the statistical calculations. P<0.05 was considered significant.
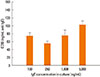
During the first 6 weeks of maturation the cells are cultured in absence of human IgE. For the last ten days of the maturation period the human mast cells were cultured with increasing concentrations of IgE. A dose-response relation was observed between the releases of histamine after the 10 days with varying amounts of IgE (Fig. 1). When no IgE is added the cells are not sensitized and no crosslinking occurs. Thus no release of histamine is observed. As the concentration of IgE is increased the histamine release increases from 26.9%±7.3% of total content at 50 ng/mL IgE until it peaks at 250 ng/mL IgE in the culture with a release of 61.8%±13.2% of total histamine content. Concentrations of IgE above 250 ng/mL result in a decrease in histamine release from 61.8%±13.2% to 21.6%±5.5% of total content (P<0.001). The large standard deviations are due to variation in maximum histamine release between donors, however the dose-response curves for all donors were similar.
The sensitivity of mast cells was compared by the EC50 value for each concentration of IgE in the culture (100, 250, 1,000, and 5,000 ng/mL) (Fig. 2). Mast cells cultured with 250 ng/mL IgE during the last 10 days of the maturation period are significantly more sensitive to activation with anti-IgE, with an EC50 value of 55.45±5.94 ng/mL than mast cells cultured with 100 (P=0.022), 1,000 (P=0.042), and 5,000 (P<0.001) ng/mL IgE giving a EC50 value of 74.39±8.24, 75.21±14.14, and 101.97±10.21 ng/mL, respectively. No significant difference in sensitivity was observed between cells cultured at other concentrations.
Although, the release of histamine and the sensitivity of the mast cells are very dependent on the IgE concentration, the total content of histamine in the cells are not under influence of the IgE concentration in the culture medium. The mean intracellular content of histamine content was 21.9±2.1 pg/cell (n=64).
The number of FcεRI on mast cell surface was dependent on the IgE concentration in culture medium. Fig. 3 shows the median and the interquartile range (IQR) of the number of FcεRI on the mast cell surface cultured with different concentrations of IgE for the last ten days of maturation. The number of FcεRI increased from 43,012 molecules/cell (IQR 30,940 -71,738) at 100 ng/mL IgE in culture medium to 292,775 (IQR 132,743-417,157 as the concentration of IgE increased to 5,000 ng/mL (P=0.01).
Another significant mediator released from activated mast cells, PGD2 does not have the same dose-response curve as observed when measuring the histamine release.
The de novo synthesis and release of PGD2 seems to be IgE concentration independent in the sense that the release of PGD2 increases with concentrations of IgE and reach a plateau at 1 ng/mL IgE in culture (Fig. 4).
As observed in histamine release, a great donor variation is observed, some mast cells release 2,000 pg/mL after FcεRI activation and other release 14,000 pg/mL, however the shape of the curves were similar between different donors. This is observed, although all batches are cultured for seven weeks under the same conditions from CD133+ cells to mature mast cells. This could indicate that there may be genetic or biological differences in the progenitor cells that influence the mediator release in the mature mast cell.
The sensitivity of the mast cell to activation through FcεRI by anti-IgE (EC50 value) was not affected by the concentration of IgE in culture medium (data not shown).
During the 7-week maturation period, the cells were counted, metachromatic stained and their morphological structure was observed weekly. Regardless of the different IgE-concentrations the number of mature mast cells obtained and metachromatic staining was not affected. At 6 week culture 88.0%±5.3% were metachromatic, and 99.6%±1.1% after 7 weeks. Neither was the morphological structure affected by the increasing IgE concentrations.
Immunostaining of the mature mast cells showed that 100% were tryptase-positive and less than 2% were positive for chymase. The expression of tryptase and chymase is independent of the IgE concentration in the culture.
The present study shows a strong association between the IgE concentration in mast cell culture medium for the last 10 days of the maturation period and the release of the preformed mediator histamine from human mast cells, while PGD2, tryptase, and chymase appear not to be affected by the increasing IgE concentrations. The cells were cultured with IgE concentrations in the range 0-5,000 ng/mL. Previous studies from Europe have shown that the probability of predicting type-I allergy is very high when total-IgE levels are above 200 IU/mL.17,18 Using the conversion factor of 2.4 (1 IU= 2.4 ng IgE)19 this means serum levels of total-IgE above 480 ng/mL. Before maturation mast cell progenitors migrate away from the bloodstream into the tissue. Since much of the IgE in tissue might be bound by IgE receptors, it is assumed that the IgE level surrounding the mast cell during the maturation is lower than serum levels. Our study showed the maximum histamine release could be observed at an IgE concentration of 250 ng/mL. This correlates with the sensitivity (from the EC50 value in Fig. 2) which also shows strong association with the IgE-concentrations and reaches its maximum at the same concentration. The increased sensitivity observed from cells cultured with 250 ng/mL IgE in culture medium causes the mast cell to degranulate at lower concentrations of anti-IgE (EC50=55.45±5.9 ng/mL anti-IgE), than cells cultured with 100 (EC50=74.39±8.2 ng/mL anti-IgE), 1,000 (EC50=75.21±14.1 ng/mL anti-IgE) or 5,000 ng/mL IgE (EC50=102.0±10.21 ng/mL anti-IgE). The increased IgE concentrations observed in patients with type-1 allergy may contribute to the increased sensitivity to allergens and thereby degranulation at lower concentration of anti-IgE as shown to be the case in this study. The release of PGD2, which is a de novo synthetized, mediator is not influenced by the IgE concentration in culture medium in the same way as observed with the release of histamine. The release of PGD2 increases at low concentrations of IgE and reaches a plateau at 1 ng/mL IgE in culture medium (Fig. 4), probably reflecting a maximum of de novo synthesis of PGD2. The lower release of PGD2 observed at concentration below 1 ng/mL IgE in culture medium may be due to insufficient saturation of the FcεRI with IgE. Contrary to the histamine release no correlation between the concentration of IgE in culture medium and the sensitivity to anti-IgE (EC50 values) were observed for the release of PGD2.
However, while the histamine release was influenced by the IgE concentration, the total histamine content of the mast cell, chymase and tryptase expression, and the release of de novo synthetized PGD2 remain unaffected by the concentration of IgE. It is not possible to tell why the increased IgE levels influences the release of histamine as described in this study. The pattern may suggest that the increased IgE concentration may influence one of the steps in the process of histamine release between the synthesis and the release by degranulation. Another suggestion to explain why the increased IgE levels influences the histamine release is that it may be due to changes in the mechanisms of cross-linking of IgE-receptors on the surface of the mast cell and thereby the activation of the mast cell.
The sensitivity of the mast cell release of histamine was also shown to be influenced by the concentration of IgE in culture medium (Fig. 2). The sensitivity increases with IgE concentration and peak at 250 ng/mL IgE in culture medium after which it decreases again. Previous studies of mast cells and other cell lines, U937 (human leukemic monocyte lymphoma cell line) and NIH3T3 (mouse embryonioc fibroblast cell line) have shown that increased IgE concentrations stabilize the FcεRI on the cell surface.8,20 The results of this study show that the same is valid for our cultured human mast cells. A higher IgE concentration resulted in a higher density of FcεRI on the human mast cell (Fig. 3). At IgE concentrations below 250 ng/mL the histamine release increases with increasing concentrations of IgE (Fig. 1). However, at concentrations of IgE above 250 ng/mL the histamine release decreases despite the increasing density of FcεRI on cell surface.
These findings are in agreement with the ideas lying in the hygiene hypothesis, which is the theory stating that infectious agents, like parasites inducing an IgE response, protect against allergic symptoms.14 The hygiene hypothesis postulates that the allergic response is modulated by parasite infections in people living in tropical rural areas. These individuals have very high serum levels of IgE despite being non-atopic, their levels might be several folds higher compared to the atopic patients.21,22
In this work human mast cells have been cultured with very high concentrations of IgE to reflect the high concentration of total-IgE observed at parasite infections.8,23 Previous studies have suggested that the lower prevalence of allergic symptoms in patients infected with parasites may be due to a saturation of FcεRI on mast cells with the huge amount of anti-parasite IgE. The anti-parasite IgE occupies the IgE-receptors on mast cells. This results in a blocking for binding of allergen-specific IgE and thereby a decrease in the allergic symptoms.9 However, our study suggests that other mechanism also may be involved in the down-regulation of allergic symptoms. We observe a negative correlation between high concentration of myelom IgE and release of histamine after an unspecific activation with anti-IgE. Thus the decreased histamine release observed at high concentration of IgE observed in this study is not due to blocking of the receptor by parasite specific IgE, but may be due to other mechanisms. The density of FcεRI increases as the concentration of IgE in culture increases and it is possible that this results in an oversaturation of the mast cell with IgE bound to the many IgE-receptors. This oversaturation might result in a sterical blocking for further binding of allergy-specific IgE to the receptors or hinder the cross-linking of 2 bound IgE molecules. Either of these sterical blockings may cause the release of mediators to reach a plateau despite the number of FcεRI increases with the increasing concentration of IgE in mast cell culture.
Figures and Tables
Fig. 1
The release of histamine from human mast cells is related to the concentration of IgE in culture medium in a dose-dependent manner (n=10).

Fig. 2
The sensitivity of mast cells to anti-IgE after culture with different concentrations of IgE (n=8). The EC50 value is the concentration of anti-IgE giving a release of histamine that is 50% of the maximum histamine release.
ACKNOWLEDGMENTS
We would like to thank Ellen-Margrethe Raaby for her persistent and excellent technical work in our laboratory.
References
1. Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008; 8:310–315.
2. Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009; 124:639–646.
3. Bansal G, Xie Z, Rao S, Nocka KH, Druey KM. Suppression of immunoglobulin E-mediated allergic responses by regulator of G protein signaling 13. Nat Immunol. 2008; 9:73–80.
4. Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007; 7:93–104.
5. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997; 77:1033–1079.
6. Siles RI, Hsieh FH. Allergy blood testing: a practical guide for clinicians. Cleve Clin J Med. 2011; 78:585–592.
7. Baldacci S, Omenaas E, Oryszczyn MP. Allergy markers in respiratory epidemiology. Eur Respir J. 2001; 17:773–790.
8. Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001; 167:1290–1296.
9. King CL, Ottesen EA, Nutman TB. Cytokine regulation of antigen-driven immunoglobulin production in filarial parasite infections in humans. J Clin Invest. 1990; 85:1810–1815.
10. Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa-Atta L, Solé D, Carvalho EM. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol. 2000; 123:145–148.
11. Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, Nutman TB. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003; 111:995–1000.
12. Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, Cruz AA, Simoes SM, Fiaccone R, Amorim L, Cooper PJ, Barreto ML. Social Change, Asthma and Allergy in Latin America. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008; 38:1769–1777.
13. van den Biggelaar AH, Rodrigues LC, van Ree R, van der Zee JS, Hoeksma-Kruize YC, Souverijn JH, Missinou MA, Borrmann S, Kremsner PG, Yazdanbakhsh M. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004; 189:892–900.
14. Andersen HB, Holm M, Hetland TE, Dahl C, Junker S, Schiøtz PO, Hoffmann HJ. Comparison of short term in vitro cultured human mast cells from different progenitors - peripheral blood-derived progenitors generate highly mature and functional mast cells. J Immunol Methods. 2008; 336:166–174.
15. Holm M, Andersen HB, Hetland TE, Dahl C, Hoffmann HJ, Junker S, Schiøtz PO. Seven week culture of functional human mast cells from buffy coat preparations. J Immunol Methods. 2008; 336:213–221.
16. Skov PS, Mosbech H, Norn S, Weeke B. Sensitive glass microfibre-based histamine analysis for allergy testing in washed blood cells. Results compared with conventional leukocyte histamine release assay. Allergy. 1985; 40:213–218.
17. Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011; 10:128–134.
18. Carosso A, Bugiani M, Migliore E, Antò JM, DeMarco R. Reference values of total serum IgE and their significance in the diagnosis of allergy in young European adults. Int Arch Allergy Immunol. 2007; 142:230–238.
19. Woszczek G, Kowalski ML, Borowiec M. Association of asthma and total IgE levels with human leucocyte antigen-DR in patients with grass allergy. Eur Respir J. 2002; 20:79–85.
20. Bazaral M, Hamburger RN. Standardization and stability of immunoglobulin E (IgE). J Allergy Clin Immunol. 1972; 49:189–191.
21. Fishbein AB, Fuleihan RL. The hygiene hypothesis revisited: does exposure to infectious agents protect us from allergy? Curr Opin Pediatr. 2012; 24:98–102.
22. Levin ME, Le Souëf PN, Motala C. Total IgE in urban Black South African teenagers: the influence of atopy and helminth infection. Pediatr Allergy Immunol. 2008; 19:449–454.
23. Cooper PJ, Alexander N, Moncayo AL, Benitez SM, Chico ME, Vaca MG, Griffin GE. Environmental determinants of total IgE among school children living in the rural tropics: importance of geohelminth infections and effect of anthelmintic treatment. BMC Immunol. 2008; 9:33.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download