Abstract
Purpose
Interleukin (IL)-13, a Th2-type cytokine, plays a pivotal role in the pathogenesis of asthma through its direct effects on airway smooth muscles. A naturally occurring IL-13 polymorphism, R110Q, is strongly associated with increased total serum IgE levels and asthma. In the present study, we aimed to determine whether the IL-13 R110Q variant would display different biochemical properties or altered functions in comparison with wild-type (WT) IL-13 in cultured human bronchial smooth muscle cells (hBSMCs).
Methods
Culture supernatants and cell proteins were collected from cultured hBSMCs that were treated with 50 ng/mL IL-13 or IL-13 R110Q for 24 hours. Eotaxin released into hBSMC culture medium was determined by ELISA. The expression levels of the high-affinity IgE receptor (FcεRI) α-chain, smooth muscle-specific actin alpha chain (α-SMA), smooth muscle myosin heavy chain (SmMHC), and calreticulin in the cells were measured on Western blots.
Asthma, which is characterized by airway inflammation and hyperresponsiveness, is a major public health problem in children worldwide, with an increased incidence over the past few decades.1,2 Despite significant research into the pathogenesis of asthma, specific pathways underlying its development remain controversial. IL-13, a Th2-type cytokine, has been reported to play a pivotal role in the pathogenesis of asthma. Increased levels of IL-13 are correlated with allergic diseases, such as allergic rhinitis, dermatitis, atopy, and asthma.3-6 IL-13 has also been shown to participate in the regulation of airway inflammation, mucosal secretions, airway hyperresponsiveness, and bronchoconstriction.7-9
The bronchial smooth muscle (BSM), an important tissue in the bronchial tree, is involved in the regulation of airway caliber and bronchomotor tone.10 Traditionally viewed as a passive contractile tissue, BSM cells has been shown to be active participants in modulating inflammation and hyperresponsiveness in asthma.9 Evidence suggests that IL-13 may directly interact with the airway smooth muscle (ASM) to produce the deleterious effects of asthma.
Many proteins involved in smooth muscle function have been linked to IL-13. These include the smooth muscle actin alpha chain (α-SMA) and smooth muscle myosin heavy chain (SmMHC), both of which are vital for smooth muscle contraction.11 Calreticulin, a calcium regulating protein, has important roles in the homeostatic control of calcium levels in the cytosol and sarcoplasmic reticulum.12 The high-affinity receptor for IgE (FcεRI) participates in the IgE-dependent signaling pathway; the activation of FcεRI is involved in airway inflammation/hyperresponsiveness and intracellular calcium mobilization.13 Eotaxin is a chemoattractant for eosinophils, basophils, and Th2-like lymphocytes. The recruitment of these immune cells to the airways by eotaxin results in airway hyperresponsiveness and local inflammation.14
IL-13 has been reported to stimulate eotaxin release, regulate smooth muscle contractile protein expression, and increase calcium signaling and contractile responses in ASM.7,15,16 It also participates in multiple signaling pathways in cultured ASM cells through the phosphorylation of signal transducer and activator of transcription 6 (STAT-6), extracellular signal-regulated kinase (ERK) as well as mitogen-activated protein kinases (MAPKs), and through the activation of NF-κB.8,17,18
The naturally occurring IL-13 polymorphism R110Q leads to amino acid changes from arginine to glutamine at residue 110.19,20 The IL-13 R110Q variant is strongly associated with increased total serum IgE levels and asthma,4,19 and functional studies have revealed different biochemical properties and altered functions in comparison with wild-type (WT) IL-13.21,22 There have been few reports on the effects of IL-13 R110Q on human ASM/BSM. Thus, we aimed to address whether the IL-13 R110Q variant would display different biochemical properties or altered functions from WT IL-13 in cultured human bronchial smooth muscle cells (hBSMCs).
Commercially available primary hBSMCs (Science Cell, USA) were maintained in smooth muscle cell medium (SMCM; Science Cell) containing 2% fetal bovine serum (FBS), 0.5 ng/mL human epidermal growth factor, 5 µg/mL insulin, 2 µg/mL human fibroblast growth factor-basic, 50 µg/mL gentamicin, and 50 ng/mL amphotericin B, as recommended by the supplier. The cells were grown at 37℃ in a humidified atmosphere with 5% CO2. The medium was completely changed every 2 days and cells were passaged when reaching 90%-95% confluence. For the treatment experiments, hBSMCs (passages 6-8) were seeded in 6-cm culture plates (Corning, NY, USA) at a density of 1×104 cells/cm. Cells at 80%-85% confluence were incubated in serum-free medium for 24 hours before stimulation with recombinant human IL-13 or IL-13 R110Q (PeproTech Inc., Rocky Hill, NJ, USA) at 1, 10, 50, and 100 ng/mL for 24 hours. The concentration range and incubation time were chosen based on previous reports.23-25 Following the treatment, culture supernatants and cell proteins were collected for enzyme-linked immunosorbent assays (ELISAs) and Western blot analyses, respectively.
To determine the effect of IL-13 and the IL-13 R110Q variant on the expression of hBSMC contractile proteins, Western blot analysis was performed. Briefly, ice-cold lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1% Na3VO4, 0.5 µg/mL leupeptin, and 1 mM phenylmethanesulfonyl fluoride (Beyotime Institute of Biotechnology, Jiangsu, China) was added directly to the cultured cells. The lysates were clarified by centrifugation at 12,000×g for 5 minutes, and protein yields were determined using a protein assay kit (Beyotime Institute of Biotechnology). Equal amounts of proteins were separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and electrotransferred to PVDF membranes. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing Tween 20 (TBST; 25 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.05% (v/v) Tween 20) at room temperature for 1 hour and then incubated with primary antibodies diluted in TBST containing 5% non-fat dry milk at 4℃ overnight. The primary antibodies used for immunoblotting were monoclonal anti-α-SMA (1:1,000; Abcam Inc., Cambridge, MA, USA), anti-calreticulin (1:1,000; Epitomics Inc., Cambridge, MA, USA), anti-SmMHC (1:500; Merck Millipore, Merck KGaA, Darmstadt, Germany), and anti-GAPDH (1:5,000; Epitomics), which was used as a loading control. Following three 10-min washes in PBS, the immunoblots were incubated with HRP-conjugated IgG secondary antibody (1:5,000; Epitomics) diluted in TBST containing 5% non-fat dry milk at room temperature for 2 hours. Immunoreactivity was detected using an ECL Detection kit (Pierce Biotechnology, Rockford, IL, USA). The optical densities of the corresponding bands were measured using Image-Pro (Media Cybernetics, Bethesda, MD, USA). Each experiment was repeated at least 3 times.
To measure the eotaxin concentration in culture supernatants, a Quantikine Human Eotaxin Immunoassay ELISA Kit (R&D Systems, Minneapolis, MN, USA) was used according to the manufacturer's instructions. Briefly, 100 µL of the assay diluent RD1W and 50 µL of the standard or sample were added to the wells of a 96-well plate and incubated at room temperature for 2 hours. The content of each well was aspirated, the wells were washed 3 times with wash buffer and 200 µL of eotaxin conjugate was added to each well. After incubation at room temperature for 1 hour, the wells were again washed 3 times with wash buffer. Substrate solution was added to each well, the plate was incubated at room temperature for 30 minutes in the dark, and the reaction was stopped with the addition of stop solution. Within 30 minutes, the optical density of each well was measured at 450 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, US).
The regulation of the chemoattractant eotaxin is poorly understood. Eotaxin release into the culture medium by hBSMCs following treatment with WT IL-13 and IL-13 R110Q was measured by ELISA. In the concentration range of 0-100 ng/mL, both IL-13 and IL-13 R110Q promoted eotaxin release in a concentration-dependent manner. The release of eotaxin was greater with IL-13 R110Q than with WT IL-13 at each concentration tested and was significantly greater at and higher concentrations of 10 ng/mL (P<0.05); the greatest difference in eotaxin release occurred at 50 ng/mL (P<0.01; Fig. 1). These results indicate that compared with WT IL-13, the IL-13 R110Q variant enhances eotaxin release from hBSMCs.
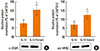
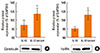
Changes in the ASM phenotype, which involves alterations in the expression levels of contractile proteins, such as α-SMA, desmin, and MHC, play a fundamental role in the pathogenesis of asthma.11,26 The expression levels of the phenotypic markers, SmMHC and α-SMA, in cultured hBSMCs treated with IL-13 and the IL-13 R110Q variant were investigated by Western blot analysis. Cells treated with IL-13 R110Q showed modest but significant increases the expression levels of α-SMA and SmMHC compared with the levels in cells treated with WT IL-13 (P<0.05; Fig. 2A and B), suggesting that the IL-13 R110Q polymorphism is associated with the increased expressions of phenotypic ASM markers.
The modulation of FcεRI and calreticulin expression in ASM represents a potentially important mechanism in asthma. Western blot analysis of the cultured hBSMCs revealed that treatment with the IL-13 R110Q variant induced modest but significant increases in the expression levels of FcεRI α-chain (P<0.05; Fig. 3A) and calreticulin (P<0.05; Fig. 3B) compared with the respective expression levels after treatment with WT IL-13.
Asthma is a complicated disease resulting from complex interactions among genetic factors, an environmental allergens, and irritants.27,28 Many genetic loci conferring susceptibility to asthma and atopy have been described and polymorphisms in these genes are involved in the development and severity of asthma.19,27,29
IL-13, an immunoregulatory cytokine, appears to play a central role in the pathogenesis of asthma. IL-13 variants have been shown to display biochemical properties and functions that differ in some respects from those of WT IL-13.21,22 Polymorphisms generally enhance the functional activities of IL-13 variants, including the induction of increased STAT6 phosphorylation and CD23 expression in monocytes and B cells,30 increased eosinophilic inflammation in the upper and lower airways of aspirin-intolerant asthma patients,31 and increased receptor activity and airway hyperresponsiveness.32 However, no differences have been noted in the expression of "pro-asthmatic" genes between cells exposed to WT IL-13 and IL-13 R130Q.24
IL-13 concentrations in the range of 1-100 ng/mL have been used to stimulate cultured hBSMCs.23-25 In the present study, 4 different concentrations of IL-13 and the IL-13 R110Q variant (1, 10, 50, and 100 ng/mL) were used to stimulate eotaxin release from hBSMCs. The difference in eotaxin release from hBSMCs treated with WT IL-13 versus from hBSMCs treated with the IL-13 R110Q variant was greatest at a concentration of 50 ng/mL (Fig. 1), in agreement with the results of previous studies.23-25 Thus, this concentration was used for IL-13 stimulation of hBSMCs in all subsequent experiments in the present study.
In addition to its enhanced effect on eotaxin release, the IL-13 R110Q variant also promoted increased expression levels of calreticulin, α-SMA, SmMHC, and FcεRI α-chain compared with the levels induced by WT IL-13. This data demonstrates that a single amino acid substitution in IL-13 can have significant biological effects on cultured hBSMCs. However, the mechanism accounting for this enhanced functional activity is not clear.
The receptor complex through which IL-13 mediates its effects contains the subunit IL-13Rα1, which binds to IL-13. This then forms a complex with the IL-4 receptor subunit IL-4Rα to initiate downstream signal transduction. A closely related IL-13 receptor subunit, IL-13Rα2, is a high-affinity receptor with a short cytoplasmic tail; it does not induce signal transduction, but may be responsible primarily for local sequestration of IL-13.21,32
No significant difference was found between the binding of R110Q or WT IL-13 to IL-13Rα.21 However, a lower affinity of IL-13Rα2 for IL-13 R110Q, enhanced plasma stability, and augmented local levels of IL-13 have been observed. Previous studies have shown that a soluble form of the IL-13Rα2-Fc chimera was unable to neutralize IL-13 R110Q as efficiently as WT IL-13.21,30 Andrew et al.33 also demonstrated a lower affinity of IL-13 R110Q for IL-13Rα2, but not for IL-13Rα1. Their research further demonstrated that the level of IL-13Rα2 regulates IL-13 R110Q-mediated responses; low levels of IL-13Rα2 significantly increase IL-13 R110Q-induced eotaxin release and STAT6 phosphorylation compared with WT IL-13-induced activities. In contrast, high levels of IL-13Rα2 or addition of an IL-13Rα2-neutralizing antibody, completely abolished the observed differences between the WT and the variant.33 The IL-13 R110Q variant was also found to increase functional activity through IL-13Rα1.32 Consequently, we hypothesize that the lower affinity of IL-13Rα2 for IL-13 R110Q leads to an increased local concentration of IL-13, resulting in increased signal transduction via IL-13Rα1.
A structural change conferred by the R110Q polymorphism is believed to be the cause of altered IL-13 function. Previous mutational analysis has shown that the mutation site is not directly involved in binding to IL-13Rα1 or IL-13Rα2. Instead, the D helix of the IL-13 molecule has been identified as an important region for the binding of IL-13 to IL-13Rα1 and IL-13Rα2.34 Recent site-directed mutagenesis experiments suggest that the substitution of glutamine for arginine at position 110 may change the conformation of the IL-13 molecule rather than disrupt a direct binding interaction with IL-13R.35 Using NMR relaxation analysis, Yoshida et al.36 confirmed that the internal motion of helix D was increased in IL-13 R110Q compared with that of WT IL-13, which may affect the binding affinity.37 Oshima et al.38 demonstrated that the substitution of an arginine residue with the negatively charged aspartic acid at residue 112 results in a 5-fold increase in affinity. Therefore, the increased internal motion on helix D in IL-13 R110Q may be involved in the lower affinity of IL-13Rα2 for IL-13 R110Q, which would increase the level of available IL-13 R110Q and possibly account for its enhanced functional activity.
IL-13 has been linked to all of the biochemical markers studied in the present work. Recent studies have identified RhoA as a critical positive regulator of smooth muscle cell contraction, which involves SmMHC, α-SMA, tropomyosin, and calponin.11 IL-13 has been shown to upregulate RhoA and to markedly enhance the contractility of cultured hBSMCs.18 Exogenous administration of IL-13 or transgenic overexpression of IL-13 in mouse lungs results in increased pulmonary expression of eotaxin, a chemoattractant for immune cells.39,40 In vivo animal models and in vitro cell culture studies also support a role of IL-13 in eotaxin release and eosinophil recruitment.14,23 The modulation of FcεRI expression in ASM is a potentially important mechanism in asthma,13,41 and IgE has been shown to upregulate FcεRI expression in human mast cells and ASM cells.13,42 Both WT IL-13 and the IL-13 R110Q variant were strongly associated with increased total serum IgE content and could thereby indirectly modulate FcεRI expression.4,19,43 Calreticulin controls Ca2+ homeostasis in the cytosol and sarcoplasmic reticulum and is affected by continuous fluctuations in the Ca2+ concentration in the sarcoplasmic reticulum.12,44 IL-13 has been reported to increase calreticulin expression, promote intracellular Ca2+ release, and increase cytosolic calcium levels in cultured ASM cells.7,17,45
The data presented here support the hypothesis that compared with WT IL-13, the IL-13 R110Q variant would enhance functional activity. The lower affinity of IL-13Rα2 for IL-13 R110Q could increase IL-13 signal transduction via IL-13Rα1, and this may be the primary mechanism mediating the enhanced functional activity of IL-13R110Q. The targeting of IL-13 and its associated receptors can be use as a potential therapeutic approach to asthma and allergies.
Figures and Tables
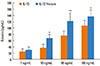 | Fig. 1hBSMCs were treated with the indicated concentrations of WT IL-13 or the IL-13 variant (1, 10, 50, and 100 ng/mL) for 24 h. Following treatment, culture supernatants were collected, and the eotaxin concentration was measured by ELISA. Data are presented as means±SD from three or more samples. |
ACKNOWLEDGMENTS
This work was supported by a National Natural Science Grant of China (No. 30972750 to Yi-xiao Bao) and a grant from the Science and Technology Commission of Shanghai Municipality (Medical Major Project No. 10DZ1951000 to Yi-xiao Bao). The authors declare no conflicts of interest.
References
1. Lee SI. Prevalence of childhood asthma in Korea: international study of asthma and allergies in childhood. Allergy Asthma Immunol Res. 2010; 2:61–64.
2. Crinnion WJ. Do environmental toxicants contribute to allergy and asthma? Altern Med Rev. 2012; 17:6–18.
3. Liu X, Nickel R, Beyer K, Wahn U, Ehrlich E, Freidhoff LR, Björkstén B, Beaty TH, Huang SK. An IL13 coding region variant is associated with a high total serum IgE level and atopic dermatitis in the German multicenter atopy study (MAS-90). J Allergy Clin Immunol. 2000; 106:167–170.
4. Heinzmann A, Mao XQ, Akaiwa M, Kreomer RT, Gao PS, Ohshima K, Umeshita R, Abe Y, Braun S, Yamashita T, Roberts MH, Sugimoto R, Arima K, Arinobu Y, Yu B, Kruse S, Enomoto T, Dake Y, Kawai M, Shimazu S, Sasaki S, Adra CN, Kitaichi M, Inoue H, Yamauchi K, Tomichi N, Kurimoto F, Hamasaki N, Hopkin JM, Izuhara K, Shirakawa T, Deichmann KA. Genetic variants of IL-13 signalling and human asthma and atopy. Hum Mol Genet. 2000; 9:549–559.
5. Benson M, Adner M, Cardell LO. Cytokines and cytokine receptors in allergic rhinitis: how do they relate to the Th2 hypothesis in allergy? Clin Exp Allergy. 2001; 31:361–367.
6. Wang M, Xing ZM, Lu C, Ma YX, Yu DL, Yan Z, Wang SW, Yu LS. A common IL-13 Arg130Gln single nucleotide polymorphism among Chinese atopy patients with allergic rhinitis. Hum Genet. 2003; 113:387–390.
7. Tliba O, Deshpande D, Chen H, Van Besien C, Kannan M, Panettieri RA Jr, Amrani Y. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br J Pharmacol. 2003; 140:1159–1162.
8. Goto K, Chiba Y, Misawa M. IL-13 induces translocation of NF-kappaB in cultured human bronchial smooth muscle cells. Cytokine. 2009; 46:96–99.
9. Ozier A, Allard B, Bara I, Girodet PO, Trian T, Marthan R, Berger P. The pivotal role of airway smooth muscle in asthma pathophysiology. J Allergy (Cairo). 2011; 2011:742710.
10. Amrani Y, Panettieri RA Jr. Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr Opin Allergy Clin Immunol. 2002; 2:39–45.
11. Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001; 90:358–368.
12. Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010; 24:665–683.
13. Gounni AS, Wellemans V, Yang J, Bellesort F, Kassiri K, Gangloff S, Guenounou M, Halayko AJ, Hamid Q, Lamkhioued B. Human airway smooth muscle cells express the high affinity receptor for IgE (Fc epsilon RI): a critical role of Fc epsilon RI in human airway smooth muscle cell function. J Immunol. 2005; 175:2613–2621.
14. Hemelaers L, Louis R. Eotaxin: an important chemokine in asthma. Rev Med Liege. 2006; 61:223–226.
15. Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001; 164:141–148.
16. Desai LP, Wu Y, Tepper RS, Gunst SJ. Mechanical stimuli and IL-13 interact at integrin adhesion complexes to regulate expression of smooth muscle myosin heavy chain in airway smooth muscle tissue. Am J Physiol Lung Cell Mol Physiol. 2011; 301:L275–L284.
17. Moynihan B, Tolloczko B, Michoud MC, Tamaoka M, Ferraro P, Martin JG. MAP kinases mediate interleukin-13 effects on calcium signaling in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008; 295:L171–L177.
18. Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol. 2009; 40:159–167.
19. Graves PE, Kabesch M, Halonen M, Holberg CJ, Baldini M, Fritzsch C, Weiland SK, Erickson RP, von Mutius E, Martinez FD. A cluster of seven tightly linked polymorphisms in the IL-13 gene is associated with total serum IgE levels in three populations of white children. J Allergy Clin Immunol. 2000; 105:506–513.
20. Leung TF, Tang NL, Chan IH, Li AM, Ha G, Lam CW. A polymorphism in the coding region of interleukin-13 gene is associated with atopy but not asthma in Chinese children. Clin Exp Allergy. 2001; 31:1515–1521.
21. Arima K, Umeshita-Suyama R, Sakata Y, Akaiwa M, Mao XQ, Enomoto T, Dake Y, Shimazu S, Yamashita T, Sugawara N, Brodeur S, Geha R, Puri RK, Sayegh MH, Adra CN, Hamasaki N, Hopkin JM, Shirakawa T, Izuhara K. Upregulation of IL-13 concentration in vivo by the IL13 variant associated with bronchial asthma. J Allergy Clin Immunol. 2002; 109:980–987.
22. Vercelli D. Genetics of IL-13 and functional relevance of IL-13 variants. Curr Opin Allergy Clin Immunol. 2002; 2:389–393.
23. Moore PE, Church TL, Chism DD, Panettieri RA Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L847–L853.
24. Syed F, Panettieri RA Jr, Tliba O, Huang C, Li K, Bracht M, Amegadzie B, Griswold D, Li L, Amrani Y. The effect of IL-13 and IL-13R130Q, a naturally occurring IL-13 polymorphism, on the gene expression of human airway smooth muscle cells. Respir Res. 2005; 6:9.
25. Chiba Y, Goto K, Misawa M. Interleukin-13-induced activation of signal transducer and activator of transcription 6 is mediated by an activation of Janus kinase 1 in cultured human bronchial smooth muscle cells. Pharmacol Rep. 2012; 64:454–458.
26. Amrani Y, Panettieri RA. Airway smooth muscle: contraction and beyond. Int J Biochem Cell Biol. 2003; 35:272–276.
27. Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res. 2010; 2:215–227.
28. Mukherjee AB, Zhang Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem. 2011; 286:32883–32889.
29. Leung TF, Chan IH, Wong GW, Li CY, Tang NL, Yung E, Lam CW. Association between candidate genes and lung function growth in Chinese asthmatic children. Clin Exp Allergy. 2007; 37:1480–1486.
30. Vladich FD, Brazille SM, Stern D, Peck ML, Ghittoni R, Vercelli D. IL-13 R130Q, a common variant associated with allergy and asthma, enhances effector mechanisms essential for human allergic inflammation. J Clin Invest. 2005; 115:747–754.
31. Palikhe NS, Kim SH, Cho BY, Choi GS, Kim JH, Ye YM, Park HS. IL-13 Gene Polymorphisms are Associated With Rhinosinusitis and Eosinophilic Inflammation in Aspirin Intolerant Asthma. Allergy Asthma Immunol Res. 2010; 2:134–140.
32. Chen W, Ericksen MB, Levin LS, Khurana Hershey GK. Functional effect of the R110Q IL13 genetic variant alone and in combination with IL4RA genetic variants. J Allergy Clin Immunol. 2004; 114:553–560.
33. Andrews AL, Bucchieri F, Arima K, Izuhara K, Holgate ST, Davies DE, Holloway JW. Effect of IL-13 receptor alpha2 levels on the biological activity of IL-13 variant R110Q. J Allergy Clin Immunol. 2007; 120:91–97.
34. Madhankumar AB, Mintz A, Debinski W. Alanine-scanning mutagenesis of alpha-helix D segment of interleukin-13 reveals new functionally important residues of the cytokine. J Biol Chem. 2002; 277:43194–43205.
35. Arima K, Sato K, Tanaka G, Kanaji S, Terada T, Honjo E, Kuroki R, Matsuo Y, Izuhara K. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem. 2005; 280:24915–24922.
36. Yoshida Y, Ohkuri T, Takeda C, Kuroki R, Izuhara K, Imoto T, Ueda T. Analysis of internal motions of interleukin-13 variant associated with severe bronchial asthma using (15)N NMR relaxation measurements. Biochem Biophys Res Commun. 2007; 358:292–297.
37. Zídek L, Novotny MV, Stone MJ. Increased protein backbone conformational entropy upon hydrophobic ligand binding. Nat Struct Biol. 1999; 6:1118–1121.
38. Oshima Y, Joshi BH, Puri RK. Conversion of interleukin-13 into a high affinity agonist by a single amino acid substitution. J Biol Chem. 2000; 275:14375–14380.
39. Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999; 162:2477–2487.
40. Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999; 103:779–788.
41. Gounni AS. The high-affinity IgE receptor (FcepsilonRI): a critical regulator of airway smooth muscle cells? Am J Physiol Lung Cell Mol Physiol. 2006; 291:L312–L321.
42. Yamaguchi M, Hirai K, Komiya A, Miyamasu M, Furumoto Y, Teshima R, Ohta K, Morita Y, Galli SJ, Ra C, Yamamoto K. Regulation of mouse mast cell surface Fc epsilon RI expression by dexamethasone. Int Immunol. 2001; 13:843–851.
43. Kabesch M, Schedel M, Carr D, Woitsch B, Fritzsch C, Weiland SK, von Mutius E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006; 117:269–274.
44. Moynihan BJ, Tolloczko B, El Bassam S, Ferraro P, Michoud MC, Martin JG, Laberge S. IFN-gamma, IL-4 and IL-13 modulate responsiveness of human airway smooth muscle cells to IL-13. Respir Res. 2008; 9:84.
45. Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol. 2004; 31:36–42.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download