Abstract
Purpose
The oil spill from the Heibei Spirit in December 2007 contaminated the Yellow Coast of South Korea. We evaluated the respiratory effects of that spill on children who lived along the Yellow Coast.
Methods
Of 662 children living in the area exposed to the oil spill, 436 (65.9%) were enrolled as subjects. All subjects completed a modified International Study of Asthma and Allergies in Childhood questionnaire. A health examination, including a skin prick test, pulmonary function test, and methacholine bronchial provocation test (MBPT), was administered. The children were assigned to two groups: those who lived close to the oil spill area and those who lived far from the oil spill area.
Results
The children who lived close to the oil spill area showed a significantly lower forced expiratory volume in one second (FEV1), an increased prevalence of 'asthma ever' (based on a questionnaire), and 'airway hyperresponsiveness' (based on the MBPT) than those who lived far from the oil spill area (FEV1; P=0.011, prevalence of 'asthma ever' based on a questionnaire; P=0.005, prevalence of 'airway hyperresponsiveness' based on the MBPT; P=0.001). The onset of wheezing after the oil spill was significantly higher in children who lived close to the oil spill area than in those who lived far from the oil spill area among the 'wheeze ever' group (P=0.002). In a multiple logistic regression analysis, male sex, family history of asthma, and residence near the oil spill area were significant risk factors for asthma (sex [male/female]: odds ratio [OR], 2.54; 95% confidence interval [CI], 1.31-4.91; family history of asthma [No/Yes]: OR, 3.77; 95% CI, 1.83-7.75; exposure group [low/high]; OR, 2.43; 95% CI, 1.27-4.65).
Oil spills pose a serious threat to marine life. Major oil spill accidents have been reported in the United Arab Emirates, Kuwait, Malaysia, India, Norway, Libya, the United States, England, Japan, France, Spain, and Pakistan.1 Only a few studies have focused on the effect of oil spills on human health, although the effect of oil spills on ecological systems has been extensively studied. Most studies regarding the human health effect of oil spills have focused on the acute toxic effects and psychological effects of oil spills on humans.1 Little is known about the effect of oil spills on the human respiratory system. A few epidemiological studies have shown an increased prevalence of respiratory symptoms in residents or clean-up workers immediately after exposure to an oil spill, which may be prolonged after the spill.2-8 The effect of oil spills on lung function has been inconsistent in several clinical studies.2,8-11 There has been only one study in children that showed no deleterious effect of exposure to an oil spill on lung function.9
On December 7, 2007, a crane barge being towed by a tug collided with an anchored crude oil carrier, the Hebei Spirit.12 The Hebei Spirit was carrying 260,000 tons of crude oil, which included Iranian heavy oil, Upper Zakum oil, and Kuwait Export oil. About 10,000 tons of crude oil was spilt into the sea and contaminated the coastline (about 167 kilometers long). The oil was composed of volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene, or xylene, polyaromatic hydrocarbons (PAHs), and heavy metals. VOCs, which typically contain 1-18 carbon atoms, are associated with indoor air pollution and adverse health effects such as respiratory tract irritation, bronchitis, and irritation to the skin.13
Recent epidemiological studies have shown that VOCs are associated with impaired lung function and an increased prevalence of asthma in children as well as in adults.13-15 PAHs, a group of small organic compounds containing three to five benzene rings, can induce oxidative stress in the respiratory tract and aggravate asthma symptoms.16-18 The aim of this study was to evaluate the respiratory effect of oil spill exposure on children.
The present study was performed to evaluate the respiratory effects of oil spill exposure on children living in the area exposed to the spill. The study protocol was approved by the Institutional Review Board of Dankook University Hospital. Of 662 children (aged 6 to 12 years) living in the area exposed to the oil spill, 436 (65.9%) who completed a questionnaire and agreed to participate in the study were enrolled. A health examination, including a skin prick test, pulmonary function test, and methacholine bronchial provocation test (MBPT), was performed. The skin prick test was performed in 418 children (95.9%); 18 children refused the test. The pulmonary function test was performed for all 436 children, and the MBPT was performed for 103 children (23.6%) who were suspected of having bronchial asthma based on their responses to the questionnaire. The health examination was performed during 10 days in June 2009. There was no control group without oil spill exposure, epidemiologic data before the accident were absent, and the concentration of air pollution at the time of the accident was not measured. Consequently, we did not directly evaluate the effects of oil spill exposure. Therefore, we divided the children into groups according to those who lived close to or far from the oil spill area (i.e., whether they lived within 2 km of the contaminated coastline [Figure]).
The Modified International Study of Asthma and Allergies in Childhood questionnaire (Korean version) was used to evaluate characteristics related to asthma.19 The prevalence of asthma (asthma ever) was determined by asking the subject whether he/she had ever been diagnosed with asthma by a doctor. The prevalence of wheezing (wheeze ever) was determined by lifetime and current (during the last 12 months) wheezing episodes. A question about whether the asthma symptoms began before or after oil spill exposure was added to the questionnaire.
The questionnaires, which were completed by the parents of the study subjects before the health examination, were collected on the day of the examination.
The skin prick test was performed in 418 children (95.9%). Subjects were tested for common inhalant allergens (house dust mites, cockroach, mixed grass pollen, mixed tree pollen, weed pollen, cat hair, and mold mixture; Allergopharma, Reinbek, Germany). The skin prick test was performed by three trained personnel at our center. The skin test result was regarded as positive when the wheal size of the allergen was more than 3 mm and larger than that of histamine.
The pulmonary function test was performed according to American Thoracic Society guidelines.20 The forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured by three trained personnel at our center using a portable micro-spirometer (Microspiro HI-298; Chest Corp., Tokyo, Japan).
The MBPT was administered using a modified version of Chai's method21 to 104 children from the asthma ever or wheeze ever group. Briefly, the FEV1 and FVC were measured using a spirometer (Microspiro HI-298; Chest Corp.), taking the largest values of triplicate FEV1 and FVC measurements. The subjects inhaled five breaths of increasing methacholine concentration until the FEV1 fell to less than 80% of its baseline value or the highest concentration of methacholine was reached. Triplicate FEV1 measurements were made starting at 90 s after each inhalation, and the largest value was used for analysis. If the concentration of methacholine that caused a 20% fall in FEV1 was less than 16 mg/mL, the subject was considered to have 'airway hyperresponsiveness.'
A statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). An independent t-test was used to compare continuous variables such as age, height, or weight, and pulmonary function test results between the study groups. A chi-squared test was used to compare categorical variables such as sex, family history of asthma, smoking, asthma ever, wheeze ever, symptoms related to asthma within 12 months, some of the skin prick test results, and the prevalence of asthma between the study groups. Fisher's exact test was used for nonparametric categorical variables such as the onset of wheezing and some of the skin prick test results. A multiple logistic regression analysis was used to evaluate risk factors for the prevalence of asthma in the study population. Statistical significance was defined as a P value less than 0.05.
The mean age of the study subjects was 9.6 years, and the ratio of males to females was 0.8 (Table 1). Children who lived close to or far from the oil spill area showed no significant differences in terms of age, sex, height, weight, and family history of asthma. Children who lived close to the oil spill area were weighted and exposed to passive smoke compared with those who lived far from the oil spill area. The percentage of children who were current smokers was similar between the two groups (Table 1). The prevalence of 'asthma ever' was 14.0%; the prevalence of 'wheeze ever' was 19.0%. In a univariate analysis for characteristics related to asthma, the number of children with previously diagnosed asthma and reported wheezing after oil spill exposure was significantly higher in children who lived close to the oil spill area than in those who lived far from the oil spill area (P=0.005 and 0.002, respectively). The number of children with wheezing-limited speech, sleep disturbances caused by wheezing, or absence from school due to wheezing within the previous 12 months was not significantly different between the groups. However, the number of children with wheezing at rest, wheezing after exercise, or treatment due to asthma within the previous 12 months was significantly higher in children who lived close to the oil spill area than in children who lived far from the oil spill area (P=0.038, 0.002, and 0.037, respectively).
House dust mites, which were positive in 101 children (24.2%), were the most common aeroallergen in the study subjects (Table 2). In decreasing order, molds were positive in 19 subjects (4.5%), tree pollen was positive in 11 subjects (2.6%), weed pollen was positive in 8 subjects (1.9%), cat hair was positive in 8 subjects (1.9%), cockroach was positive in 8 subjects (1.9%), and grass pollen was positive in 5 subjects (1.2%). However, the rate of skin test positivity showed no significant difference between the two groups.
The FEV1 was significantly lower in children who lived close to the oil spill area than in those who lived far from the oil spill area, although the FVC and FEV1/FVC ratio were not significantly different between the two groups (P=0.011; Table 3).
The prevalence of 'wheeze ever' and 'wheeze current' based on the questionnaire was significantly higher in children who lived close to the oil spill area than in those who lived far from the oil spill area (P=0.050 and 0.002, respectively; Table 1). The prevalence of 'increased airway hyperresponsiveness' based on the MBPT was also significantly higher in children who lived close to the oil spill area than in those who lived far from the oil spill area (P=0.001; Table 3). In the multiple logistic regression analysis, male sex, family history of asthma, and residence close to the oil spill area were significant risk factors for asthma (male sex: odds ratio [OR], 2.54; 95% confidence interval [CI]: 1.31-4.91; family history of asthma: OR, 3.77; 95% CI: 1.83-7.75; residence close to the oil spill area: OR, 2.43; 95% CI: 1.27-4.65; Table 4).
The present study shows that children who lived close to the oil spill area had more asthmatic symptoms, increased impaired lung function, and an increased prevalence of asthma than those who lived far from the oil spill area. The risk factors for asthma in our study subjects were male sex, family history of asthma, and residence close to the oil spill area.
Oil spill exposure has hazardous effects on marine life; however, few studies have reported the effects of oil spill exposure on human health. Particularly, little is known regarding the effect of oil spill exposure on the human respiratory system. When an oil spill occurs, local inhabitants or volunteers who participate in clean-up activities constitute a population whose health may be affected by the oil spill because they are highly exposed to the spill immediately after the accident. A few epidemiologic studies have shown acute or prolonged respiratory symptoms in residents as well as in clean-up workers exposed to an oil spill.2-8,22 However, these studies were performed in adults who lived near the oil spill area or who had participated in clean-up activities. Only one study documented the effect of oil spills on children, in which children who lived near the oil spill area did not have impaired lung function immediately after the spill.9 Our study showed increased respiratory symptoms in children who lived close to the oil spill area after the spill. This finding is consistent with previous study results showing increased respiratory symptoms in adults exposed to an oil spill.
The effect of oil spill exposure on lung function produced inconsistent results in several clinical studies. Earlier studies have shown that lung function in adults or children who lived near the oil spill area did not deteriorate immediately after the spill.2,9 Only one study on the effect of oil spill exposure in children who lived near the oil spill area did not show impaired lung function immediately after the spill.9 Conversely, our study showed that the FEV1 (%) was significantly reduced in children who lived close to the oil spill area compared to those who lived far from the oil spill area. The difference in the results between our study and Crum's study9 can be explained by the difference in time of the performance of the lung function test after the oil spill accident. Therefore, we believe that our study better reflects the long-term effects of oil spill exposure than Crum's study.9 In other studies, clean-up workers who participated in clean-up activities after oil spill exposure showed impaired lung function shortly after the oil spill, which was reversible 1 year after the spill.10,11 In a recent study of the effect of oil spill exposure on 501 clean-up workers, no significant difference in lung function was detected between clean-up workers and controls without exposure to the oil spill 2 years after the spill.8 However, makers of airway injury such as 8-isoprostane, which reflect local oxidative stress, were increased in the breath condensate of clean-up workers, and a subgroup of nonsmokers had a higher risk of bronchial hyperreactivity in a methacholine bronchial challenge.8 In our study, children who lived close to the oil spill area had increased impaired lung function than those who lived far from the oil spill area 18 months after the spill. This inconsistent finding may be due to physiologic differences between children and adults or a genetic factor, although further study is needed to determine the causes of these discrepancies.
Our study revealed that children who lived close to the oil spill area had more asthmatic symptoms, increased impaired lung function, and an increased prevalence of bronchial hyperreactivity than those who lived far from the oil spill area. However, there are limitations to our study in that a control group without oil spill exposure could not be included, and only 436 children (65.9%) were enrolled among the 662 children living in the area exposed to the oil spill. Thus, a possibility of selection bias exists. Furthermore, epidemiologic data before the accident such as asthma prevalence at the study area were absent. We could not directly assess whether the prevalence of asthma was increased after the accident at the oil spill area.
Therefore, we divided the children into those who lived close to or far from the oil spill area (i.e., whether they lived within 2 km of the contaminated coastline). Additionally, we hypothesized that children who lived far from the oil spill area (more than 2 km) were less influenced by the oil spill because there is a hill 2 km from the accident area.
The prevalence of asthma in our study subjects was compared with that in a nationwide survey performed during 2006 and 2007. The prevalence of 'asthma ever' and 'wheeze ever' in our study subjects was 14.0 and 19.0%, respectively, which is about twice as high as that in a nationwide survey of 30,893 children in Korea.23 The prevalence of asthma based on the MBPT in our study participants was 11.7%, which was more than twice as high as that in a survey of 622 children and teenagers between the ages of 7 and 19 years in Korea.24 The prevalence of asthma in Taean is higher than that in the general population. The causes of the difference in prevalence are not obvious. We suppose that the coastal climate, yellow dust, and air pollution influenced the development of asthma in the Taean area. However, despite the regional characteristics concerning oil spill exposure in Taean, our study is remarkable in that wheeze ever was increased in children who lived close to the oil spill area after the spill, and the prevalence of bronchial hyperreactivity was higher in children who lived close to the oil spill area.
The present results support those of a study that showed increased markers of airway injury and a high prevalence of bronchial hyperreactivity in clean-up workers 2 years after oil spill exposure.8 The study suggests that prolonged asthmatic symptoms after oil spill exposure may be mediated through bronchial hyperreactivity from airway inflammation induced by some irritants included in crude oil, although biologic markers related to airway inflammation were not measured in our study.
VOCs and PAHs are irritants of toxicological interest included in crude oil. Recent epidemiological studies have shown that VOCs are associated with impaired lung function and an increased prevalence of asthma in children as well as in adults.13-15 PAHs can induce oxidative stress in the respiratory tract and aggravate asthma symptoms.16-18 Acute irritant-induced asthma-also called reactive airways dysfunction syndrome-is caused by an inhalation accident in an occupational or the general environment.25 The most well-known outbreak of this syndrome occurred following the World Trade Center disaster, in which firefighters were exposed to various irritants.26 Similar to the acute irritant-asthma observed in the World Trade Center disaster, our study shows that oil spill exposure can affect the development or aggravation of acute irritant-induced asthma. Considering the results of the present study, a long-term follow-up study of the children who participated in our study will be needed to evaluate the relationship between oil spill exposure and the prevalence of asthma.
Our study results suggest that oil spill exposure is a risk factor for asthma in children. Future longitudinal studies will be needed to demonstrate the relationship between oil spill exposure and asthma and should include biologic markers related to airway inflammation to reveal the mechanism whereby oil spill exposure induces impaired lung function and increases the prevalence of asthma.
Figures and Tables
Figure
Map showing the location of the Heibei Spirit oil spill and the areas of high and low exposure according to the distance from the spill.
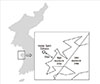
Table 1
Participant characteristics

Table 2
Skin prick test results in the study participants
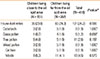
Table 3
Pulmonary function test results and the prevalence of asthma in the study participants

Table 4
Risk factors for asthma in the study participants

ACKNOWLEDGEMENTS
This study was supported by a Taean Environmental Health Center operating grant for the investigation of mid- and long term health effects of the Hebei Spirit Oil Spill from the Ministry of Environment, Republic of Korea.
References
1. Aguilera F, Méndez J, Pásaro E, Laffon B. Review on the effects of exposure to spilled oils on human health. J Appl Toxicol. 2010; 30:291–301.
2. Campbell D, Cox D, Crum J, Foster K, Christie P, Brewster D. Initial effects of the grounding of the tanker Braer on health in Shetland. The Shetland Health Study Group. BMJ. 1993; 307:1251–1255.
3. Lyons RA, Temple JM, Evans D, Fone DL, Palmer SR. Acute health effects of the Sea Empress oil spill. J Epidemiol Community Health. 1999; 53:306–310.
4. Suárez B, Lope V, Pérez-Gómez B, Aragonés N, Rodríguez-Artalejo F, Marqués F, Guzmán A, Viloria LJ, Carrasco JM, Martín-Moreno JM, López-Abente G, Pollán M. Acute health problems among subjects involved in the cleanup operation following the Prestige oil spill in Asturias and Cantabria (Spain). Environ Res. 2005; 99:413–424.
5. Janjua NZ, Kasi PM, Nawaz H, Farooqui SZ, Khuwaja UB, Najamul H, Jafri SN, Lutfi SA, Kadir MM, Sathiakumar N. Acute health effects of the Tasman Spirit oil spill on residents of Karachi, Pakistan. BMC Public Health. 2006; 6:84.
6. Campbell D, Cox D, Crum J, Foster K, Riley A. Later effects of grounding of tanker Braer on health in Shetland. BMJ. 1994; 309:773–774.
7. Zock JP, Rodríguez-Trigo G, Pozo-Rodríguez F, Barberá JA, Bouso L, Torralba Y, Antó JM, Gómez FP, Fuster C, Verea H. SEPAR-Prestige Study Group. Prolonged respiratory symptoms in clean-up workers of the prestige oil spill. Am J Respir Crit Care Med. 2007; 176:610–616.
8. Rodríguez-Trigo G, Zock JP, Pozo-Rodríguez F, Gómez FP, Monyarch G, Bouso L, Coll MD, Verea H, Antó JM, Fuster C, Barberá JA. SEPAR-Prestige Study Group. Health changes in fishermen 2 years after clean-up of the Prestige oil spill. Ann Intern Med. 2010; 153:489–498.
9. Crum JE. Peak expiratory flow rate in schoolchildren living close to Braer oil spill. BMJ. 1993; 307:23–24.
10. Meo SA, Al-Drees AM, Meo IM, Al-Saadi MM, Azeem MA. Lung function in subjects exposed to crude oil spill into sea water. Mar Pollut Bull. 2008; 56:88–94.
11. Meo SA, Al-Drees AM, Rasheed S, Meo IM, Khan MM, Al-Saadi MM, Alkandari JR. Effect of duration of exposure to polluted air environment on lung function in subjects exposed to crude oil spill into sea water. Int J Occup Med Environ Health. 2009; 22:35–41.
12. Sim MS, Jo IJ, Song HG. Acute health problems related to the operation mounted to clean the Hebei Spirit oil spill in Taean, Korea. Mar Pollut Bull. 2010; 60:51–57.
13. Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health. 2007; 80:711–719.
14. Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004; 59:746–751.
15. Elliott L, Longnecker MP, Kissling GE, London SJ. Volatile organic compounds and pulmonary function in the Third National Health and Nutrition Examination Survey, 1988-1994. Environ Health Perspect. 2006; 114:1210–1214.
16. Leem JH, Kim JH, Lee KH, Hong Y, Lee KH, Kang D, Kwon HJ. Asthma attack associated with oxidative stress by exposure to ETS and PAH. J Asthma. 2005; 42:463–467.
17. Suresh R, Shally A, Mahdi AA, Patel DK, Singh VK, Rita M. Assessment of association of exposure to polycyclic aromatic hydrocarbons with bronchial asthma and oxidative stress in children: a case control study. Indian J Occup Environ Med. 2009; 13:33–37.
18. Miller RL, Garfinkel R, Lendor C, Hoepner L, Li Z, Romanoff L, Sjodin A, Needham L, Perera FP, Whyatt RM. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatr Allergy Immunol. 2010; 21:260–267.
19. Hong SJ, Kim SW, Oh JW, Rah YH, Ahn YM, Kim KE, Koh YY, Lee SI. The validity of the ISAAC written questionnaire and the ISAAC video questionnaire (AVQ 3.0) for predicting asthma associated with bronchial hyperreactivity in a group of 13-14 year old Korean schoolchildren. J Korean Med Sci. 2003; 18:48–52.
20. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.
21. Kim YK, Kim SH, Tak YJ, Jee YK, Lee BJ, Kim SH, Park HW, Jung JW, Bahn JW, Chang YS, Choi DC, Chang SI, Min KU, Kim YY, Cho SH. High prevalence of current asthma and active smoking effect among the elderly. Clin Exp Allergy. 2002; 32:1706–1712.
22. Meo SA, Al-Drees AM, Rasheed S, Meo IM, Al-Saadi MM, Ghani HA, Alkandari JR. Health complaints among subjects involved in oil cleanup operations during oil spillage from a Greek tanker "Tasman Spirit". Int J Occup Med Environ Health. 2009; 22:143–148.
23. Suh M, Kim HH, Sohn MH, Kim KE, Kim C, Shin DC. Prevalence of allergic diseases among Korean school-age children: a nationwide cross-sectional questionnaire study. J Korean Med Sci. 2011; 26:332–338.
24. Kim KM, Kwon HS, Jeon SG, Park CH, Sohn SW, Kim DI, Kim SS, Chang YS, Kim YK, Cho SH, Min KU, Kim YY. Korean ginseng-induced occupational asthma and determination of IgE binding components. J Korean Med Sci. 2008; 23:232–235.
25. Malo JL, L'archevêque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med. 2009; 179:923–928.
26. Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, Cosenza K, Christodoulou V, Glass L, Al-Othman F, Weiden MD, Kelly KJ, Prezant DJ. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010; 362:1263–1272.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download