Abstract
Food allergy has increased dramatically in prevalence over the past decade in westernized countries, and is now a major public health problem. Unfortunately for patients with food allergy, there is no effective therapy beyond food allergen avoidance, and rapid medical treatment for accidental exposures. Recently, oral immunotherapy (OIT) has been investigated as a treatment for this problem. In this review, we will discuss the progress in developing OIT for food allergy, including a novel approach utilizing Xolair (anti-IgE monoclonal antibody, omalizumab) in combination with OIT. This combination may enhance both the safety and efficacy of oral immunotherapy, and could lead to a widely available and safe therapy for food allergy.
Food allergy, which results from an exaggerated and inappropriate immune response to certain foods, has become a major public health problem in westernized countries, and also a major source of anxiety and stress for both affected children and their parents. In the US, the overall prevalence of food allergy has been estimated to be 3.5%1 and has recently witnessed at least an 18% increase from 1997 to 2007, particularly in young children.2 The most common allergenic foods in children and their estimated prevalence in North America are: milk (2.5%), egg (1.5%), peanuts (1%), tree nuts (0.5%), shellfish (0.1%), fish (0.1%), and wheat/soy (0.4%).3-6 Definitive and accurate assessment of the prevalence of food allergy however, is limited by the different definitions of food allergy and the variability of diagnostic methods utilized in various studies.7
While food allergy can be divided broadly into IgE-mediated and non-IgE-mediated disease, in this review, we will focus on IgE-mediated food allergy, which can manifest as urticaria, angioedema, wheezing and respiratory distress, vomiting, diarrhea, cardiovascular collapse and anaphylaxis generally occurring with 1-60 minutes after exposure. Non-IgE-mediated food allergy manifests as celiac disease, food protein-induced enteropathy, eosinophilic gastroenteritis or lactose intolerance. With IgE-mediated diseases however, the clinical presentation, degree of severity, and natural course of food allergy as well as the frequency of reactions vary widely among different individuals and even within the same individual. Nevertheless, across all ages, food allergy represents the most common cause of anaphylaxis seen in the emergency department and is associated with increasing hospitalizations and rarely death.8-11 Allergy to peanuts and tree nuts has been shown to be associated with more severe anaphylactic reactions,8,9 and a lower chance of spontaneous resolution when compared to milk and egg allergies.
Currently, there is no effective, definitive and curative therapy for food allergy. The standard therapeutic approach consists of allergen avoidance, nutritional counseling and rapid medical treatment in case of accidental exposure. In this review, we will discuss several therapeutic approaches for food allergy that are currently being investigated. In particular, we will discuss oral immunotherapy (OIT) and a novel approach utilizing a combination of Xolair (omalizumab) and OIT.
One can subdivide IgE-mediated food allergy into three subtypes: transient, persistent, and oral allergy syndrome (also known as pollen associated food allergy syndrome).12 Each form differs from the others in terms of prognosis, and each has a different therapeutic approach.
Oral allergy syndrome is manifested mainly by oral symptoms (lips, mouth, and throat) that occur after ingestion of certain fresh foods of plant origin in patients with allergic rhinitis/pollen allergy. It is thought to be due to immunologic cross-reactivity between plant pollen allergens and proteins found in certain related fruits and vegetables.13 Patients with transient food allergy usually outgrow their allergy over time, and therefore food avoidance is generally the recommended treatment, although it is possible that immunotherapy may hasten food allergy resolution. Allergy to cow's milk, egg, wheat and soy in children are generally transient, with 80% resolving by age 16 years. In contrast, persistent food allergy usually starts in childhood and persists into adulthood. Allergy to peanut, tree nuts, fish, and shellfish are usually persistent, and only about 20% of patients outgrow peanut/tree nut allergy. Distinguishing transient from persistent forms of food allergy is critical early on for instituting appropriate therapeutic strategies, particularly since patients with persistent food allergy may be candidates for desensitization therapies. However, some patients with persistent food allergy manifest a less favorable therapeutic response including failure of desensitization and/or achievement of oral tolerance (see below). Note that the distinction between transient and persistent food allergy is not always clear. While foods like milk and egg tend to be associated with transient food allergy, over the past decade these food allergies have persisted in individuals for greater time periods, for reasons that are not fully understood. In addition, several studies have suggested that higher levels of baseline food specific IgE may be useful for identifying persistent food allergy in patients with milk or egg allergy.14 This concept might also be applicable to peanut or tree nut allergy.
The classical approach to food allergy is complete avoidance of the offending allergen. In many patients with transient food allergy, there is a hope that the food allergy can be outgrown over time. However, this allergen avoidance approach is extremely challenging for patients and their families and requires constant vigilance and active precautions. Adherence is particularly difficult with foods that are common, ubiquitous ingredients, resulting in a high risk of accidental exposure, or with foods that are not clearly included in labels.15,16 Because of the required vigilance and possible risk of reactions this treatment modality has a negative impact on the emotional, psychological and social wellbeing of children and their families.17
The cross reactivity between major allergenic epitopes of pollens and those of certain foods has suggested a role for specific immunotherapy with certain pollens (e.g., birch) in patients with oral allergy syndrome. Subjects treated with subcutaneous pollen immunotherapy or sublingual immunotherapy (SLIT) have experienced variable beneficial effects on their oral symptoms and skin test reactivity to certain plant foods.13,18-21 These effects were predominantly reported in adults mono-sensitized to birch tree pollen and treated with high dose pollen immunotherapy. In 49 adults with birch-induced allergic rhinitis and oral allergy syndrome to apple, a significant reduction (50% to 95%) or complete resolution of apple-induced oral symptoms was demonstrated in 84% of treated subjects compared with no benefit in control subjects (P<0.001). Additionally, a reduction in skin test reactivity to fresh apple was seen in 88% of these subjects after 12 months of birch immunotherapy.13 Although 18 months post cessation of birch immunotherapy the majority of subjects reverted to positive skin prick test (SPT) responses, more than 50% could tolerate ingestion of apple. In another study, on the other hand, SLIT with birch pollen extract did not significantly reduce apple-induced oral allergy syndrome symptoms in adults with birch-induced allergic rhinitis.22 Therefore, treatment recommendations for oral allergy syndrome remains controversial.
Treatment of egg and milk proteins with high temperatures denatures allergenic proteins rendering them less allergenic. This observation has led to OIT using heated food proteins.23,24 Several studies have shown that most allergic subjects treated with heated (baked) egg/milk tolerated the heated food protein (70% of children with egg allergy and 75% of children with milk allergy). Although some food proteins show enhanced allergenicity with heating, as is the case with peanuts,25 treatment of egg or milk allergic patients with baked forms of these foods is an appealing alternative to strict food avoidance. This approach may hasten the resolution of transient food allergy, but may not be effective for patients with severe food allergy, or those with high milk-specific IgE.
For patients with persistent food allergy, several different forms of immunotherapy are currently being explored. SLIT with food allergens is one of these approaches, first reported in 2003.26 In this approach, the food is administered sublingually, held in the mouth for few minutes, and then spit or swallowed. Several studies, with hazelnut, milk, peanut, and peach have demonstrated the benefit of SLIT in increasing the amount of the food tolerated on double-blind placebo-controlled food challenge (DBPCFC).27-30 Side effects are generally mild, primarily limited to oropharyngeal symptoms, and rarely requiring oral antihistamine administration. However, the maximum dose that can be administered sublingually is limited, which may limit the maximum dose of food that can be ultimately tolerated. Recent studies suggest that combining SLIT with subsequent OIT (see below) may benefit from the safety profile of SLIT and potential for achieving higher doses of food with OIT. Further studies are needed to standardize the method and demonstrate its safety in larger numbers of patients.
Another approach for persistent food allergy is epicutaneous immunotherapy (EIT), in which patients receive three 48-hour skin patch applications (1 mg of skimmed milk powder) per week for 3 months. Such an approach was studied in small randomized placebo controlled pilot study with 18 children with cow's milk allergy (mean age, 3.8 years; age range, 10 months to 7.7 years).31 Subjects in the active treatment group consistently showed higher doses of tolerated milk on food challenges during follow up visits when compared to subjects in the placebo group (increase of threshold doses at follow-up oral milk challenge. In both groups at 3 months, cow's milk-specific IgE levels did not change significantly from baseline. Adverse effects consisted mostly of local cutaneous reactions and discomfort (pruritis and eczema) and repeated doses of diarrhea in one child but did not include any severe systemic reactions. While EIT appears safe, clearly, additional studies are required to examine efficacy in terms of additional foods, and what are the maximum doses that can be applied epicutaneously and tolerated orally.
The use of subcutaneous immunotherapy (SCIT) in persistent food allergy was abruptly discontinued after reports of fatal reactions with peanut injections. A double-blind placebo-controlled trial32 with 12 patients assigned in a 1 to 1 ratio to either peanut extract or control showed that SCIT with peanut was effective, although a high rate of severe systemic reactions was noted in the treatment group. Consequently, this approach is no longer used.
OIT is currently the most actively and extensively investigated approach for persistent food allergy. This method, in which the food is usually given orally starting a low doses and increasing at variable rates, is based on the assumption that oral/intestinal exposure to antigens normally leads to tolerance, and on many years of experience with protocols for oral antibiotic desensitization. Oral food immunotherapy protocols usually include an initial rapid dose escalation phase, followed by a slower build up phase to reach the desired maintenance dose. Over the past eight years, many studies (discussed below, and in Table) have shown that OIT is effective and reasonably safe, although allergic reactions occur in the majority of treated patients.
Several studies have demonstrated that OIT with milk is effective in desensitizing patients with cow's milk allergy. In 2004, Meglio et al.33 reported on a 6 month protocol of OIT in milk allergic patients with the goal of enabling subjects to tolerate a daily dose of 200 mL (6,700 mg) of cow's milk (CM). 21 patients, aged 5 to 10 years, diagnosed with milk allergy either by means of DBPCFC or by a strong positive history of reaction to CM, were enrolled. The doses ranged from 0.06 mg of CM protein to a maximum of 200 mL. Three of the 21 enrolled patients failed because of allergic reactions after minimal amounts of diluted milk. 15 of the subjects (71.4%) were fully desensitized (daily intake of 200 mL of CM), and 3 (14.3%) were partially desensitized (daily intake of 40-80 mg of CM).
In a placebo controlled clinical trial by Skripak et al.,34 20 patients, aged 6 to 17 years, with IgE-mediated milk allergy, were randomly assigned in a 2 to 1 ratio to milk powder or placebo powder. The mean milk specific IgE level at enrollment was 34.8 kUA/L (range 4.86-314 kUA/L) and 14.6 kUA/L (range 0.93-133.4 kUA/L) for active and placebo groups respectively. All patients underwent baseline DBPCFC. The study was divided into 3 phases: 1) Rapid dose escalation with an initial dose of 0.4 mg of milk protein and subsequent doubling doses administered every 30 minutes up to a maximum of 50 mg (cumulative dose of 98.7 mg) and a minimum of 12 mg (cumulative dose of 23.7 mg). 2) Home dosing with an initial dose corresponding to the maximum dose tolerated on the escalation day and followed by a dose increase from 75 mg to 500 mg every 1 to 2 weeks. 3) Maintenance phase: daily consumption of 500 mg (15 mL of milk) for a period of 13 weeks. A second DBPCFC, preformed at the end of the maintenance phase, showed an increase in the threshold of sensitivity to milk in the active treatment group from a baseline median of 40 mg to post desensitization median of 5,100 mg (range 2,540 to 8,140 mg) whereas no change was observed in the control group.
In a larger placebo controlled trial, Longo et al.35 enrolled 60 patients with severe milk allergy, all with milk specific IgE levels above 85 kUA/L. Patient, aged 5 to 17 years, were randomly assigned, in a 1 to 1 ratio, to receive either gradually increasing amounts of cow's milk (1 part of cow's milk and 9 parts of an amino acid-based infant formula modified with vanilla flavor) or placebo. Subjects underwent a baseline DBPCFC and were eligible to enter the study if they reacted at doses of 0.8 mL or less of cow's milk. The protocol consisted of an in hospital 10-day rush phase during which increasing doses of diluted milk were given each day. The home phase consisted of a 1 mL increase every second day and the percentage dose increase was adjusted to meet every person's level of confidence and was guided by the severity and frequency of adverse events. After one year, 36% were successfully desensitized (daily intake of 150 mL or more of cow's milk), 54% were partially desensitized (daily intake of 5 mL to 150 mL) and 10% withdrew because of allergic side effects. None of the subjects in the control group was able to tolerate 5 mL of cow's milk. These results suggested that OIT could work in many but not all patients with severe milk allergy.
A recent open-label randomized study done by Keet et al.,36 compared the efficacy and safety of SLIT and OIT. 30 patients with IgE-mediated cow's milk allergy, aged 6 to 17 years, were randomly assigned to SLIT alone or to SLIT followed by OIT. Initially, all study subjects received SLIT alone for a minimum of 4 weeks up to a dose of 3.7 mg. Dose escalation was subsequently continued with randomization into 3 groups: 1) SLIT with a goal dose of 7 mg and a minimum of 7 updosing visits; 2) OIT A with a goal of 2 g orally and a minimum of 19 updosing visits; 3) OIT B with a goal of 1 g orally with a minimum of 19 updosing visits. A second DBPCFC was performed 12 weeks after achieving the target dose and was followed by a 48 weeks of daily maintenance therapy. An open oral food challenge to 8 g of cow's milk protein followed. Fifteen of the 30 enrolled subjects were able to pass the final 8 g oral challenge (1/10 in the SLIT group; 8/10 in the OIT A group and 6/10 in the OIT B group). Thus, this study showed that SLIT + OIT is more effective than SLIT alone in achieving desensitization most likely due to the much higher cumulative allergen dose achieved in the SLIT + OIT group.
At the end of the desensitization phase, the investigators in this study elegantly examined for the development of tolerance (see below). The 15 subjects who passed the DBPCFC after the desensitization period underwent a trial of 1 and 6 weeks off oral maintenance therapy. One week off of therapy, 2 subjects lost tolerance. Six weeks off of therapy 3/8 in OIT A group and 3/6 in OIT B group lost tolerance. The loss of desensitization in these patients suggests that a longer maintenance treatment protocol (>48 weeks) may be required to ensure the development of tolerance. However, the lowest reaction threshold in the six subjects who lost tolerance occurred at about 75 mL of milk, which is much higher than that of the 1st food challenge at the start of therapy (median <5 mL) and well above most accidental exposures. This finding indicates that although desensitization can be lost after 6 weeks of milk avoidance, patients were still relatively protected against accidental exposures.
In a study of egg OIT, Buchanan et al.37 enrolled 7 children with egg allergy, median age 48 months. The modified rush phase started with 0.1 mg of egg, followed by doubling doses every 30 minutes. During the build up phase, the maximal tolerated dose at the end of the rush phase was given, followed by biweekly increases of 25 mg until 150 mg was reached. At that point, increases of 50 mg were administered at each visit until the final goal dose of 300 mg was reached. This maintenance dose was then given daily at home, for a period of 24 months. A DBPCFC was done at the end of the OIT protocol and showed a significant increase in the tolerated doses of egg protein compared to the rush phase and an increase in the mean cumulative dose of egg protein associated with adverse reactions from 0.05 g at the beginning of the study to 11.93 at the end (P=0.008). Limitations of this study were its small cohort size, the absence of a history of severe clinical reaction to egg ingestion at recruitment and the lack of a control group and therefore the possibility of occurrence of spontaneous natural tolerance. Although the 300 mg maintenance dose was not enough to induce long-term tolerance in all patients, it was enough to protect against most accidental exposures to egg.
Peanut OIT may be a greater challenge than milk or egg OIT, since peanut allergy usually does not resolve spontaneously, and more severe reactions may occur. In an open label study of peanut OIT by Jones et al.38 39 subjects, aged 1 to 16 years, with baseline IgE levels >7 kU/L and a clinical reaction within the previous 6 months were enrolled. The study was divided into 3 phases: 1) Initial escalation day with a starting dose of 0.1 mg of peanut protein followed by doubling doses every 30 minutes up to 50 mg; 2) build up phase: starting with the highest dose tolerated at the end of escalation phase and followed by gradual spaced dose increases up to 300 mg (25 mg increase every 2 weeks for subjects who have reached 50 mg at the end of escalation; doubling doses every 2 weeks to reach 50 mg followed by 25 mg increases for those who could not reach the desired 50 mg at the end of escalation phase); 3) maintenance phase of variable length with a median of 4.7 months (range, 4-22 months). 10 patients (25%) withdrew from the study after the dose escalation phase for either personal reasons or allergic reactions to OIT. In a post-maintenance OFC, 27 of the 29 remaining subjects (93%) were able to ingest a total dose of 3.9 g of protein whereas the remaining 2 subjects stopped at doses lower or equal to 2.1 g of peanut protein.
Another trial of peanut OIT was recently described by Blumchen et al.39 23 patients with a median age of 5.6 years and median baseline peanut-specific IgE levels of 95.6 kUA/L were enrolled. Peanut was administered at a starting dose equivalent to 1/100 of the eliciting reaction dose during an initial DBPCFC and the dose was gradually increased over a period of 7 days, at a frequency of 2 to 4 times a day (Rush phase). Patients who failed to achieve the top 500 mg peanut dose in this period of time were given incremental doses every 2 to 4 weeks (build up phase) until the highest dose up to 500 mg was achieved. All patients continued on maintenance phase for 8 weeks, followed by complete peanut avoidance for 2 weeks. Final DBPCFC showed a median 4 fold increase in the tolerated amount of peanut when compared to baseline OFC. Out of the 23 enrolled patients, 14 patients were fully desensitized tolerating a peanut dose of 0.5 to 2 g and 1 patient was partially desensitized (0.2 g). The remaining 8 patients dropped out of the study (1 during rush phase for anxiety and 7 during long-term build-up protocol due to side effects and compliance issues).
The combined results of these studies indicate that OIT for food allergy is effective in increasing the amount of food tolerated in approximately 50% to 75% of the treated patients.33-35,37-39 A subset of patients however, appears to be resistant to oral desensitization: 10%-20% of patients withdraw due to adverse reactions, and another 10%-20% achieves only partial desensitization. This suggests that patients are heterogeneous, and that some patients are easier to desensitize than others.
Identification of patients who may be resistant to desensitization may be important since these protocols are being considered for use in community clinical practice. Therefore, these patients, who often develop significant allergic reactions, might be excluded from community-based protocols. High levels of food specific IgE may identify some of these patients, since in the peanut OIT study conducted by Blumchen et al.,39 patients who failed to reach the goal of 500 mg had a median peanut specific IgE of 212 kUA/L (range: 14.3-2,071 kUA/L), whereas patients who tolerated 500 mg of peanut or more had a median baseline peanut-specific IgE of 9.1 kUA/L (range: 2.97-27 kUA/L). Similarly, the 3 patients, in Meglio's milk OIT study,33 who failed desensitization and experienced severe adverse reactions with minimal amounts of milk, were found to have the highest baseline allergen-specific IgE (class 4 or 5 for casein and/or β-lactoalbumin). This correlation however, is not absolute, as one of the fully desensitized patients had a class of level 5 of milk-specific IgE. In addition, in the egg OIT study conducted by Buchanan et al.,37 the study subject who had the highest IgE level was successfully desensitized and was able to pass the first food challenge without any adverse effects.
Most patients experienced at least one adverse event over the course of the study. Reactions varied in intensity from mild discomfort to severe reactions that required treatment and occasional subject withdrawal from the study. No life threatening event or death has been reported in any of the OIT protocols so far. Although reactions occurred at all times during the study period, the majority took place during the initial rush phase/rapid escalation phase. Importantly, the frequency and severity of side effects may be lower when prophylactic antihistamines were administered,33,35 by not having a rush phase, by having longer escalation or maintenance phases, or by omitting subjective symptoms scoring (Table).
Although previous studies have demonstrated short-term efficacy with induced desensitization, data on the long-term efficacy of immunotherapy is limited. Moreover, whether desensitization will lead to tolerance, as defined by the ability of the patient to stop oral intake of the food for a specified length of time and later pass an oral challenge to the food, is not yet clear. The development of tolerance, as defined in this way, may suggest "cure", although the "specified length of time" currently is quite short, on the order of weeks to a few months. In the study by Staden et al.,40 in which children with milk or hen's egg allergy received either OIT or elimination diet, the persistence of oral tolerance in subjects who were successfully desensitized by OIT was evaluated by elimination diet for a period of 2 months. Only 36% of the desensitized subjects developed tolerance, which was comparable to the rate in the elimination group (natural tolerance).
A shorter period of elimination diet of 2 weeks was used by Blumchen et al.39 as part of their protocol on peanut oral immunotherapy. A DBPCFC done at the end of the elimination diet showed that 11 of the 14 desensitized patients tolerated the same or higher amounts of peanut compared to the maximal achieved maintenance dose, and the remaining 3 tolerated less. Similarly, Buchanan et al.37 tested tolerance and long term efficacy of their protocol on egg OIT by performing a DBPCFC following a 3 to 4 months period off egg. Two of the 4 patients who had passed the first challenge, passed the second with no reaction.
One of the best studies of tolerance was that by Keet et al.36 of milk desensitized patients. In this study, the patients received 48 weeks of daily oral maintenance treatment before stopping oral maintenance for 6 weeks. Surprisingly, only 9 of 15 patients remained desensitized after the 6-week period off of maintenance milk therapy.
These results together suggest that oral desensitization does not quickly lead to tolerance, even when the defining period off of the food is short (e.g., two weeks). Although some individuals may develop tolerance quickly, it is possible that a longer period of daily maintenance treatment will be required for most of the patients to develop tolerance, similar to the maintenance period for SCIT for inhaled allergens or for bee venom (>3-5 years).
A goal of the experimental studies of OIT is to develop a safe protocol that can be performed in routine clinical practice without too much concern for allergic reactions. While the current experimental results indicate that OIT is still associated with significant allergic reactions, which can often occur unexpectedly, some physicians have started OIT protocols in community settings. This has generated some controversy for several reasons, as discussed below.
The most important concern is that the best, safest and most efficacious clinical protocol has not been established. Comparison of the safety and efficacy profile of the various trials is extremely difficult, because of the heterogeneity of the protocols utilized in different studies. Differences in protocol lengths and design, differences in measurement tools and proof of existence of true food allergy, heterogeneous and complex patient population, degree of follow up, natural spontaneous tolerance especially in studies that lacked a placebo group as well as a possibility of enrollment of food-tolerant patients because of a lack of baseline DBPCFC in some studies, can all together make comprehensive comparisons of the different protocols extremely difficult. Therefore, there is a need for more studies with larger numbers of patients recruited in each study in order to increase the evidence to support broadened use of OIT for food allergy.
Selecting patients for OIT based on the presence of allergy documented by history and laboratory parameters is not sufficient to ensure the success and safety of OIT. Because of the safety issues and the length of the protocol, patients and their families must be extremely compliant, reliable and committed to the protocol. Moreover, because there may be a long maintenance therapy period, the patient must be motivated and have a strong desire to eat the food as part of their normal diet. Dedicating appropriate time for participation and follow up is very challenging due to scheduling and life styles constraints, but is required for successful completion of OIT.
Despite the relatively good results of oral immunotherapy, a large fraction of the patients fail desensitization. Delineating predictive factors for a response to OIT early on is important for choosing and guiding therapy. Factors as age at entry, baseline levels of allergen specific IgE, presence of atopic disease and other food allergies may carry prognostic significance. In addition, OIT has been studied primarily in the context of IgE-mediated food allergy, and patients with complicated IgE-mediated and non-IgE-mediated disease may not respond well to OIT.
In addition, because OIT protocols are lengthy and require close medical monitoring for anaphylaxis, they are expensive, causing concern for health insurance coverage. To date, OIT protocols are considered by many to be experimental and risky, and are also not approved in the US by the FDA. Therefore, the procedure will be difficult to disseminate until it receives FDA approval and health insurance coverage.
As discussed above, because current protocols for OIT are lengthy and associated with significant side effects, with some patients not responding, additional protocols are being examined to address the problems of safety and unresponsiveness to desensitization. A number of preclinical approaches are being considered, including use of adjuvants to more quickly induce "protective" immune responses and better antigens with reduced allergenicity. The use of mutated recombinant proteins instead of native food allergens can decrease the risk of adverse reactions during immunotherapy because of decreased IgE-binding activity. A protective immune response may be further enhanced by coadministration of the mutated epitopes with bacterial adjuvants such as nonpathogenic strains of heat-killed Escherichia coli.41,42 A mixture of different herbs used in Traditional Chinese Medicine has also been shown to have a protective effect in murine models of peanut-induced anaphylaxis43-46 and is currently being investigated in human clinical trials (using a simplified herbal formula FAHF-2, Food Allergy Herbal Formula 2).47 In addition, a protocol combining OIT with treatment with anti-IgE monoclonal antibody has been recently examined, as discussed below.
Anti-IgE monoclonal antibodies: Omalizumab is a recombinant humanized monoclonal IgE-blocking antibody. Its decreases or prevents the allergic response triggered by IgE molecules, by binding to the constant domains of free circulating IgE molecules, preventing by means of steric hindrance, binding to high affinity (FcεRI) and low affinity (FcεRII) receptors on basophils and mast cells reducing IgE-mediated mast cell and basophil degranulation on allergen exposure.48-50 By greatly reducing the level of circulating free IgE (although levels of circulating bound IgE increases) omalizumab also decreases IgE from biding to FcεRI on dendritic cells. Moreover, omalizumab treatment down regulates FcεRI expression on basophils, mast cells and dendritic cells.51 The reduced expression of FcεRI on dendritic cells is thought to reduce allergen presentation to T cells, followed by a reduction in Th2 cell activation, and a consequent decreased production of Th2 cytokines during the effector phases of allergic diseases.52
Because omalizumab does not bind to the variable allergen specific region of IgE molecules, it is an allergen non-specific modality of treatment. Subcutaneous injections of omalizumab have been shown to have relatively few and tolerable side effects mainly at the injection site. Less common observed reactions included bronchospasm, hypotension, syncope, urticaria, angioedema and rarely anaphylaxis.53-55
Omalizumab has been shown to be effective in reducing symptoms and the steroid requirements of allergic asthma, particularly in patients with moderate to severe asthma, and was approved by the FDA in 2003 for the treatment of moderate to severe asthma not responsive to other conventional treatments.48 In addition, although not approved by the FDA for this indication, omalizumab is also effective in reducing symptoms in patients with seasonal allergic rhinitis.56
Omalizumab has also been studied in combination with rush SCIT for allergic rhinitis. Such a combination was shown to increase the safety and efficacy of ragweed rush immunotherapy when compared to immunotherapy alone.57 Patients, aged 18 to 50 years were randomized in a 1:1:1:1 ratio to 4 groups: 1) omalizumab and immunotherapy; 2) omalizumab only; 3) immunotherapy only; 4) placebo. Omalizumab was administered subcutaneously at a minimum dose of 0.016 mg/kg/IgE (IU/mL)/mo every 2 or 4 weeks, depending on weight and baseline IgE levels. Patients who received omalizumab were found to have less adverse events and a 5-fold decrease in the risk of anaphylaxis, as well as a significant improvement in severity scores during ragweed season when compared to those receiving immunotherapy alone. The addition of omalizumab was therefore thought to significantly improve the safety profile of rush immunotherapy, and to permit more rapid and higher doses of allergen immunotherapy.54 However, because rush immunotherapy has not been traditionally used in clinical practice for allergic rhinitis, and because omalizumab is not FDA approved in the US for this indication, omalizumab enabled rush immunotherapy for hay fever has not been utilized in clinically practice.
Another humanized anti-IgE mAb, TNX-901 is similar to omalizumab in binding free IgE. TNX-901 was shown to improve the dose of peanut tolerated during an oral peanut challenge, suggesting that it might be effective in preventing allergic symptoms on accidental ingestion of peanuts. In this study, 84 patients with peanut allergy were randomly assigned, in a 3:1 ratio, to receive 150 mg, 300 mg, or 450 mg of TNX-901 or placebo subcutaneously every 4 weeks for 4 doses. DBPCFC were performed at the start and at the end of the study and results were compared. Prior to peanut challenge, patients tolerated an average up to 436 mg of peanut flour, whereas after treatment with the highest dose of TNX-901, patients tolerated six times more peanuts. This study suggested that treatment with anti-IgE mAb could reduce the likelihood of anaphylaxis on accidental peanut ingestion, but it did not assess the role of anti-IgE therapy on enhancing desensitization to peanuts.58
A similar phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial using omalizumab was initiated to replicate the findings with TNX-901 in peanut allergy. Patients with a history of immediate reaction to peanut, positive SPT to peanut and/or detectable peanut specific (IgE >0.35 kUA/L) were randomly assigned, in a 2:1 ratio, to receive either omalizumab or placebo every 2 to 4 weeks for 20 to 22 weeks with the minimum dose of omalizumab/study drug being 0.016 mg/kg/IgE (IU/mL) every 4 weeks. DBPCFC were conducted at the beginning and at the end of the study. However, the study was halted because of two severe anaphylactic reactions that occurred during the first DBPCFC, before omalizumab administration. Therefore, only 14 subjects were able to reach the study's primary endpoint. Six (44%) of omalizumab treated patients and three (20%) of placebo treated patients could tolerate >1,000 mg of peanut flour during the post therapy food challenge at 24 weeks. Although data was limited, there appeared to be statistical significance in the increase in maximum tolerable peanut dose from baseline pre therapy values in omalizumab treated group when compared to placebo treated group.59
Since omalizumab can reduce allergic symptoms that occur with peanut administration, a phase I pilot study was recently performed assessing the safety and efficacy of combining omalizumab treatment with OIT in young children with significant cow milk allergy. The long term goal of the study was to improve the safety of and reduce the length of time required for effective cow's milk OIT. Eleven patients, aged 7 to 17 years, with a significant history of IgE-mediated cow milk allergy including anaphylaxis were enrolled at two centers with US Food and Drug Administration approval. Milk allergy was documented by history of acute clinical reaction upon exposure to milk, positive skin test to milk (median wheal/flare 20/50 mm; wheal/erythema diameter, range 11-45/20-52 mm), high milk-specific IgE levels (median 50 kUA/L; range, 41.6-342 kUA/L) and elevated total serum IgE (median 349 kU/L; range, 148-2,593 kU/L).
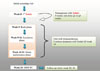
The study was divided into three phases: 9 weeks of omalizumab pretreatment; 7 to 11 weeks of oral desensitization with omalizumab treatment; 9 weeks of maintenance OIT off of omalizumab before a DBPCFC at week 24 (Figure). All patients received open-label omalizumab (anti-IgE mAb; Xolair; Genentech, San Francisco, CA, USA) with a dosing schedule based on the package insert for children with IgE levels below 700 kU/L. For children with total serum IgE levels >700 kU/L, the dose was approximately 0.016 mg/kg/IgE U/mL (225 to 300 mg) every 2 weeks.
The desensitization phase was further subdivided into two phases: a rush phase occurring over 6 hours and a slower escalation phase (weeks 9 to 16). The rush phase began with the administration of 0.1 mg of cow's milk protein followed by 11 incremental doses every 30 minutes and reaching a maximum dose of 1,000 mg (cumulative dose 1,992 mg). The subsequent dose escalation phase consisted of the highest dose of milk achieved during the rush desensitization given daily for one week, followed by weekly increase of 12.5%, with doses given daily over the course of each week. During the initial one-day rush phase, 9 of the11 subjects reached the maximal dose of 1,000 mg (cumulative dose 1,992 mg). One subject voluntarily withdrew from the study due to abdominal migraines. Two subjects experienced adverse reactions during the process: 1 subject developed, after the 1,000 mg dose (cumulative dose 1,990 mg), nasal obstruction and generalized urticaria refractory to diphenhydramine and cetirizine and was treated with epinephrine; another had symptoms at the 7 mg dose. During the slower escalation phase, 9 out of 10 remaining subjects reached the maximal daily dose of 2,000 mg and the subject who previously received epinephrine, reached a dose of 1,200 mg. After the desensitization, all subjects were instructed to continue daily milk intake (maintenance phase), and the omalizumab was discontinued.
On week 24, a DBPCFC with a top dose of 3,000 mg (cumulative dose 7,250 mg) was performed. Allergic reactions occurring during the protocol were graded based on the scoring system developed by Bock et al.60 All 9 patients who reached the dose of 2,000 mg during desensitization phase, passed the challenge and were instructed to continue with daily milk ingestion >8,000 mg/day.61 The one patient who was only able to reach a dose of 1,200 mg by the 7th week in dose escalation phase developed generalized urticaria and rhinitis at the 2,000 mg dose during the DBPCFC, and was treated with antihistamines. He was continued on a dose of 1,000 mg/day, and was later able to increase his daily dose to 4,000 mg (120 mL/day).
This study, the first to use omalizumab in combination with milk OIT, demonstrated that such a combination approach is safe and feasible, and might allow for faster desensitization of patients with food allergy, without an increase in allergic reactions. In this study, allergic reactions associated with the desensitization were primarily mild and no serious life-threatening events were observed. Importantly, the 9 of 9 patients who completed the desensitization were able to include large amounts of milk in their diets. By week 24 in the study, the 10th patient could take >1,000 mL of milk, and this dose has increased gradually over time.
Despite showing that this procedure is relative safe and efficacious, this study does have limitations: small sample size, absence of a DBPCFC at study initiation and absence of a placebo group. However, based on the results of this pilot study, this approach with omalilzumab has been extended, and is now being studied in three different studies at three institutions in patients with milk allergy (Mt. Sinai), with peanut allergy (at Duke University and at Children's Hospital Boston, Harvard Medical School). In the peanut study at Children's Hospital Boston, subjects aged 7 to 25 years, with severe IgE-mediated peanut allergy are being enrolled, with patients having peanut specific IgE >20 kUA/L, total IgE values between 50 and 2,000 kU/L and a reaction to a DBPCFC at a peanut dose of 100 mg or less. Omalizumab is being administered subcutaneously once every 2 or 4 weeks for a period of 19 weeks, with oral desensensitization occurring in the last 7 weeks of the omalizumab treatment. The primary outcome is the ability to tolerate a dose of 500 mg (cumulative 1,000 mg) following the first day of desensitization and a dose of 4,000 mg following 7 weeks (build up phase) of OIT.
In the peanut study at Duke University, the primary objective is to test whether the addition of omalizumab to peanut OIT can lead to subject desensitization in a safer, faster and more effective manner, and whether it can achieve long term tolerance. Subjects aged 12 years and above with a history of significant reaction to peanut, SPT to peanut with a wheal diameter of >3 mm and peanut-specific IgE >5 kUA/L are eligible. Omalizumab will be administered as pretreatment drug before OIT and will be kept until one month after maintenance phase. OIT will consist of 2 days of initial desensitization followed by a 4 month build up phase. Subjects will then be randomly assigned to either 12 or 24 months of maintenance with a goal of tolerating 8,000 mg of peanut powder. Desensitization will be tested by a post maintenance OFC and tolerance will be tested by a post 4 weeks elimination diet OFC.
In the study at Mt. Sinai, the aim is to compare the efficacy and safety of a combination of omalizumab and OIT with OIT alone. Subjects will be randomized to receive either omalizumab or placebo. DBPCFC will be performed at several times during the study. At the end of the study, the percentage of desensitized individuals in each arm of the study will be calculated and results will be compared. Furthermore, tolerance will be evaluated in those who have achieved desensitization after complete milk discontinuation for a certain period of time.
The studies summarized above have shown that OIT can successfully desensitize a large number of patients without major morbidity or mortality. Data on the risk of life threatening events though, is limited and longer follow up of a bigger sample size is needed before ascertaining overall long term safety of immunotherapy. At the end of all of the studies, patients can tolerate more of the food than at the start. Successful completion of OIT reduces the risk of serious reaction on accidental ingestion, and in some studies, particularly the one with omalizuamb, many patients can tolerate normal amounts of the food in their diet without symptoms. It should be emphasized however, that in all of the published studies, the enrolled study patient populations have been highly motivated, and willing to accept the risks of reactions in these experimental protocols. While the ultimate goal is to extend OIT protocols to the general public as a standard medical therapy, it is clear that OIT protocols require absolute and full patient cooperation. Although in some protocols, prevention of serious allergic reactions on accidental ingestion is the goal, in studies with omalizumab, in which the goal is to extend the diet to include a food that was previously proscribed and possibly never tasted, patient motivation is an extremely important consideration.
While achieving desensitization to a food to which the patient was previously allergic can dramatically improve the quality of life of patients, it is not yet clear if a full "cure" of food allergy can be achieved with OIT. The few studies that have examined "tolerance" have shown that only a fraction of patients develop tolerance and only when the food avoidance period after successful desensitization is short (generally <2 months). Whether tolerance can be observed after much longer periods of food avoidance after successful desensitization is yet to be determined. Practically speaking however, it may not be necessary to have very long periods of food avoidance, if the goal of OIT is to add the food to the diet, even in small quantities.
Nevertheless, even without tolerance, OIT could offer a reasonable new therapy for patients with food allergy. Future studies however, must focus on developing a standardized and safe protocol that is safe and reasonably easy to perform in the community. The final protocol will need to formalize specific entry criteria, treatment dosages, maintenance dosages and optimal followup. For patients with severe disease and high allergen-specific IgE, inclusion of anti-IgE monoclonal antibodies may be useful. Investigators and clinicians need to weigh risks and benefits of specific interventional approaches, reach at a combined decision with patients and families and be committed to ensuring the success and safety of the suggested protocol. It is important to carefully assess all parameters and individualize each patient's treatment accordingly and guided by scientific evidence.
Figures and Tables
Figure
Study protocol: Rapid oral desensitization in combination with Omalizumab (xolair) therapy in patients with cow's milk allergy. DBPCFC, double blind placebo controlled food challenge.
Table
Safety of oral immunotherapy

References
1. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009. 124:1549–1555.
2. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008. (10):1–8.
3. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010. 125:S116–S125.
4. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007. 120:638–646.
5. Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, Sodergren E, Dahlstrom J, Lindner T, Sigurdardottir ST, McBride D, Keil T. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008. 121:1210–1218.
6. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004. 113:805–819. quiz 820.
7. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011. 127:594–602.
8. Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001. 107:191–193.
9. Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007. 119:1016–1018.
10. Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007. 62:91–96.
11. Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, Bellolio MF, Bergstralh EJ, Stead LG, Li JT. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008. 122:1161–1165.
12. Nowak-Węgrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011. 127:558–573. quiz 574-5.
13. Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998. 28:1368–1373.
14. Wood RA. The natural history of food allergy. Pediatrics. 2003. 111:1631–1637.
15. Pieretti MM, Chung D, Pacenza R, Slotkin T, Sicherer SH. Audit of manufactured products: use of allergen advisory labels and identification of labeling ambiguities. J Allergy Clin Immunol. 2009. 124:337–341.
16. Joshi P, Mofidi S, Sicherer SH. Interpretation of commercial food ingredient labels by parents of food-allergic children. J Allergy Clin Immunol. 2002. 109:1019–1021.
17. Cummings AJ, Knibb RC, Erlewyn-Lajeunesse M, King RM, Roberts G, Lucas JS. Management of nut allergy influences quality of life and anxiety in children and their mothers. Pediatr Allergy Immunol. 2010. 21:586–594.
18. Asero R. How long does the effect of birch pollen injection SIT on apple allergy last? Allergy. 2003. 58:435–438.
19. Bolhaar ST, Tiemessen MM, Zuidmeer L, van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CA, Taams LS, Knol EF, van Hoffen E, van Ree R, Knulst AC. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004. 34:761–769.
20. Alonso R, Enrique E, Pineda F, Basagaña M, San Miguel-Moncín MM, Bartra J, Palacios R, Cisteró-Bahíma A. An observational study on outgrowing food allergy during non-birch pollen-specific, subcutaneous immunotherapy. Int Arch Allergy Immunol. 2007. 143:185–189.
21. Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, Prem N, Lidholm J, Ballmer-Weber BK, Vieths S, Bohle B. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011. 127:616–622.e1.
22. Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, Ebner C, Bohle B. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007. 119:937–943.
23. Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008. 122:342–347. 347.e1–347.e2.
24. Lemon-Mulé H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008. 122:977–983.
25. Gruber P, Becker WM, Hofmann T. Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. J Agric Food Chem. 2005. 53:2289–2296.
26. Mempel M, Rakoski J, Ring J, Ollert M. Severe anaphylaxis to kiwi fruit: Immunologic changes related to successful sublingual allergen immunotherapy. J Allergy Clin Immunol. 2003. 111:1406–1409.
27. Enrique E, Pineda F, Malek T, Bartra J, Basagaña M, Tella R, Castelló JV, Alonso R, de Mateo JA, Cerdá-Trias T, San Miguel-Moncín Mdel M, Monzón S, García M, Palacios R, Cisteró-Bahíma A. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005. 116:1073–1079.
28. Enrique E, Malek T, Pineda F, Palacios R, Bartra J, Tella R, Basagaña M, Alonso R, Cisteró-Bahíma A. Sublingual immunotherapy for hazelnut food allergy: a follow-up study. Ann Allergy Asthma Immunol. 2008. 100:283–284.
29. de Boissieu D, Dupont C. Sublingual immunotherapy for cow's milk protein allergy: a preliminary report. Allergy. 2006. 61:1238–1239.
30. Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, Steele P, Kamilaris J, Vickery B, Burks AW. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011. 127:640–646.
31. Dupont C, Kalach N, Soulaines P, Legoué-Morillon S, Piloquet H, Benhamou PH. Cow's milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010. 125:1165–1167.
32. Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997. 99:744–751.
33. Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE-mediated cow's milk allergy. Allergy. 2004. 59:980–987.
34. Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, Matsui EC, Burks AW, Wood RA. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008. 122:1154–1160.
35. Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, Ventura A. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol. 2008. 121:343–347.
36. Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, Steele P, Driggers S, Burks AW, Wood RA. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012. 129:448–455. 455.e1–455.e5.
37. Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, Steele PH, Pons L, Helm RM, Lee LA, Burks AW. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007. 119:199–205.
38. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, Shreffler WG, Steele P, Henry KA, Adair M, Francis JM, Durham S, Vickery BP, Zhong X, Burks AW. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009. 124:292–300. 300.e1–300.e97.
39. Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, Shreffler WG, Sampson HA, Niggemann B, Wahn U, Beyer K. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010. 126:83–91.e1.
40. Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007. 62:1261–1269.
41. Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, Sampson HA. Persistent protective effect of heat-killed Escherichia coli producing "engineered," recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003. 112:159–167.
42. Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003. 170:3289–3295.
43. Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, Schofield BH, Sampson HA. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001. 108:639–646.
44. Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, Goldfarb J, Sampson HA, Li XM. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005. 115:171–178.
45. Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007. 37:846–855.
46. Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009. 123:443–451.
47. Wang J, Patil SP, Yang N, Ko J, Lee J, Noone S, Sampson HA, Li XM. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Ann Allergy Asthma Immunol. 2010. 105:75–84.
48. Kopp MV. Omalizumab: Anti-IgE therapy in allergy. Curr Allergy Asthma Rep. 2011. 11:101–106.
49. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005. 115:459–465.
50. MacGlashan DW Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997. 158:1438–1445.
51. Lin H, Boesel KM, Griffith DT, Prussin C, Foster B, Romero FA, Townley R, Casale TB. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004. 113:297–302.
52. Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009. 64:1728–1736.
53. Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, Bousquet J, Kerstjens HA, Fox H, Thirlwell J, Cioppa GD. Omalizumab 011 International Study Group. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004. 34:632–638.
54. Kuehr J, Brauburger J, Zielen S, Schauer U, Kamin W, Von Berg A, Leupold W, Bergmann KC, Rolinck-Werninghaus C, Gräve M, Hultsch T, Wahn U. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002. 109:274–280.
55. Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann KC, Sieder C, Stenglein S, Seyfried S, Wahn U. DUAL study group. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009. 39:271–279.
56. Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, McAlary M, Fowler-Taylor A, Racine A, Gupta N, Fick R, Della Cioppa G. Omalizumab Seasonal Allergic Rhinitis Trail Group. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001. 286:2956–2967.
57. Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, Mokhtarani M, Seyfert-Margolis V, Asare A, Bateman K, Deniz Y. Immune Tolerance Network Group. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006. 117:134–140.
58. Leung DY, Sampson HA, Yunginger JW, Burks AW Jr, Schneider LC, Wortel CH, Davis FM, Hyun JD, Shanahan WR Jr. Avon Longitudinal Study of Parents and Children Study Team. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003. 348:986–993.
59. Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, Wong DA. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011. 127:1309–1310.e1.
60. Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, Bush RK, Metcalfe DD. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988. 82:986–997.
61. Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. 2011. 127:1622–1624.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download