Abstract
A 69-year-old female patient visited the emergency room with fever (38.3℃) and dyspnea. She had been taking prednisolone (5 mg once per day) and methotrexate (2.5 mg once per week) for rheumatoid arthritis for 2 years. Chest computed tomography (CT) showed bilateral, multifocal ground glass opacity with interlobular septal thickening. Peripheral blood leukocyte count was 6,520/mm3 (neutrophils, 77.4%; eosinophils, 12.1%). During the night, mechanical ventilation was initiated due to the development of severe hypoxemia. Bronchoalveolar lavage fluid showed a high proportion of eosinophils (49%). Her symptoms improved dramatically after commencement of intravenous methylprednisolone therapy. This is the first report of idiopathic acute eosinophilic pneumonia developing in a current user of systemic corticosteroids.
Acute eosinophilic pneumonia (AEP) is characterized by acute development of respiratory symptoms, such as cough, shortness of breath, and fever with eosinophilic lung infiltration.1 In most cases, the cause of AEP is unknown. Some investigators have suggested that it is an acute hypersensitivity reaction to inhaled antigens.2 Typical cases of AEP were reported among young males with recent onset of smoking.3 Many patients with AEP show progressive respiratory failure without correct diagnosis and proper treatment. Fortunately, patients with AEP are uniformly responsive to intravenous or oral corticosteroid therapy.4 The response is usually so dramatic that if a patient fails to respond to corticosteroids, an alternative diagnosis should be considered. Therefore, it is very unusual to suspect AEP when acute respiratory disease develops in an older, non-smoking female currently taking systemic corticosteroids. Here, we present a case of AEP that resulted in acute respiratory failure in a 69-year-old female patient with rheumatoid arthritis who had been taking prednisolone (5 mg once a day) regularly for 2 years.
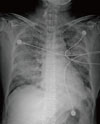
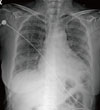
A 69-year-old female patient visited the emergency room with fever and dyspnea. She had been taking prednisolone (5 mg once per day) and methotrexate (2.5 mg once per week) for treatment of rheumatoid arthritis for 2 years. Five days prior to presentation, she developed cough and sputum, and experienced shortness of breath and fever the previous day. Upon arrival, her body temperature was 38.3℃. Plain chest X-ray and chest CT (Fig. 1) showed peribronchial consolidation and ground-glass opacity in both lungs with interlobular septal thickening. Peripheral blood leukocyte count was 6,520/mm3 (neutrophils, 77.4%; eosinophils, 12.1%). High sensitivity CRP was 78.2 mg/L (normal; 0-1.0 mg/L) and Erythrocyte sedimentation rate (ESR) was 47 mm/hr (normal; 0-20 mm/hr). Arterial blood gas analysis on room air showed severe hypoxemia (pH 7.44, PaCO2 36.2 mmHg, PaO2 43.4 mmHg, and HCO3 24.7 mmol/L). After oxygen was supplied via a facial mask with a reservoir bag at 8 L/min, PaO2 increased to 76.4 mmHg. Based on suspected community-acquired pneumonia, she was admitted to hospital and intravenous levofloxacin was started. During the night, her dyspnea worsened and arterial oxygen tension dropped below 60 mmHg while on oxygen by facial mask with the reservoir bag at 10 L/min. Follow-up chest PA showed acutely increased opacity in both lungs (Fig. 2). Endotracheal intubation was performed and mechanical ventilation was started. Bronchoscopy was performed to identify etiological pathogens of pneumonia. Bronchoalveolar lavage (BAL) fluid showed a high proportion of eosinophils (eosinophils, 49%; lymphocytes, 35%; neutrophils, 16%). Culture of bronchial washing fluid did not show growth of any significant respiratory pathogens. Methylprednisolone was started (62.5 mg intravenously every 6 hours) with a diagnosis of AEP. Prone position ventilation was applied because hemoglobin saturation of arterial blood (SaO2) barely exceeded 90% while receiving 100% oxygen. Her condition improved dramatically after introduction of systemic corticosteroids and mechanical ventilation was discontinued on the fourth day. Chest PA taken on that day showed marked improvement of her previous lung infiltration (Fig. 3). The differential eosinophil count in the blood dropped to zero the day after commencement of intravenous corticosteroid therapy. She was almost fully recovered and discharged on hospital day 9.
AEP was first reported as a cause of idiopathic acute respiratory failure.1 Several causes of AEP have been reported, such as cigarette smoking and inhalation of fire smoke.5-7 A temporal relation between the development of AEP and recent onset of cigarette smoking has been described.5 In one patient, a cigarette smoking challenge led to symptoms identical to AEP, increase in sputum eosinophils, and worsening of pulmonary function.6 AEP developed in one firefighter following collapse of the World Trade Center towers in New York in 2001.7 Our patient was a housewife and had been swimming regularly for several years. She had never smoked and had no history of recent initiation of cigarette smoking among her family members. She had lived in the same house for a long period and had not traveled abroad recently. Similar to many other cases, the cause of AEP was unknown in this case. Her current medications, prednisolone (5 mg once per day), methotrexate (2.5 mg once per week), sulfasalazine (500 mg twice per day), and hydroxychloroquine (200 mg twice per day), did not seem to be related to the development of AEP. In contrast, current use of systemic corticosteroids and immunosuppressive agents are indications against a diagnosis of AEP.
Acute hypoxemic respiratory failure requiring mechanical ventilation is a frequent presentation of AEP.8 It took less than 20 hours from arrival in the emergency room to the initiation of mechanical ventilation in this case. Interleukin-5 (IL-5) may play an important role in the pathogenesis of acute inflammation because high levels of IL-5 have been found in BAL fluid in AEP.9 IL-5 is a potent chemotaxin for eosinophils, causes the release of eosinophil granules, and prolongs eosinophil survival by inhibiting apoptosis. Early suspicion and prompt bronchoscopy are essential for the appropriate treatment of AEP. However, routine laboratory studies are nonspecific and generally unhelpful in diagnosis. In a recent study, the fraction of exhaled nitric oxide (FeNo) was shown to be significantly elevated in patients with AEP.10 FeNo may be a useful diagnostic tool to differentiate AEP from other cause of acute lung infiltration. However, FeNo is not readily available and so was not used in this case. Patients with AEP usually present with initial neutrophilic leukocytosis rather than eosinophilia, which makes early suspicion more difficult.11 Although peripheral blood eosinophilia was detected in the blood of the present patient, it was not a strong stimulus for us to proceed with bronchoscopy. Rather, it prompted us to attempt to identify respiratory pathogens of community-acquired pneumonia. Fortunately, rapid handling of BAL fluid, including differential white blood cell count, led to an early correct diagnosis of AEP and subsequent treatment with systemic corticosteroids. The response to systemic corticosteroids is so dramatic that lack of response can actually exclude AEP from the list of possible diagnoses.4 Although the patient had been taking a maintenance dose of prednisolone for the treatment of rheumatoid arthritis for 2 years, her response to intravenous methylprednisolone was also dramatic. It is very unusual for AEP to develop in a patient taking prednisolone regularly, even at such a low dose.
To our knowledge, this is the first report of idiopathic AEP leading to acute respiratory failure in a patient taking systemic corticosteroids.
Figures and Tables
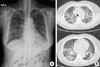
Fig. 1
Chest PA and chest CT taken on the day of arrival (hospital day 0). (A) Chest PA showed bilateral diffuse lung infiltration. (B) Chest CT demonstrated peribronchial consolidations and ground-glass opacities in the upper and lower zones of both lungs. Interlobular septal thickenings were observed at both the upper lobe and basal lungs.
References
1. Badesch DB, King TE Jr, Schwarz MI. Acute eosinophilic pneumonia: a hypersensitivity phenomenon? Am Rev Respir Dis. 1989; 139:249–252.
2. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989; 321:569–574.
3. Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002; 166:1235–1239.
4. Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med. 1999; 160:1079–1100.
5. Watanabe K, Fujimura M, Kasahara K, Yasui M, Myou S, Kita T, Watanabe A, Nakao S. Acute eosinophilic pneumonia following cigarette smoking: a case report including cigarette-smoking challenge test. Intern Med. 2002; 41:1016–1020.
6. Bok GH, Kim YK, Lee YM, Kim KU, Uh ST, Hwang JH, Kim DW. Cigarette smoking-induced acute eosinophilic pneumonia: a case report including a provocation test. J Korean Med Sci. 2008; 23:134–137.
7. Rom WN, Weiden M, Garcia R, Yie TA, Vathesatogkit P, Tse DB, McGuinness G, Roggli V, Prezant D. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002; 166:797–800.
8. Buchheit J, Eid N, Rodgers G Jr, Feger T, Yakoub O. Acute eosinophilic pneumonia with respiratory failure: a new syndrome? Am Rev Respir Dis. 1992; 145:716–718.
9. Yamaguchi S, Okubo Y, Hossain M, Fujimoto K, Honda T, Kubo K, Sekiguchi M, Takatsu K. IL-5 predominant in bronchoalveolar lavage fluid and peripheral blood in a patient with acute eosinophilic pneumonia. Intern Med. 1995; 34:65–68.
10. Lee JE, Rhee CK, Lim JH, Lee SM, Shim YS, Lee CT, Lee SW. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. 2012; 141:1267–1272.
11. Miki K, Miki M, Nakamura Y, Suzuki Y, Okano Y, Ogushi F, Ohtsuki Y, Nakayama T. Early-phase neutrophilia in cigarette smoke-induced acute eosinophilic pneumonia. Intern Med. 2003; 42:839–845.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download