Abstract
Purpose
The environmental factors human rhinoviruses (HRVs) and house dust mites (HDMs) are the most common causes of acute exacerbations of asthma. The aim of this study was to compare the chemokine production induced by HRVs in airway epithelial cells with that induced by other respiratory viruses, and to investigate synergistic interactions between HRVs and HDMs on the induction of inflammatory chemokines in vitro.
Methods
A549 human airway epithelial cells were infected with either rhinovirus serotype 7, respiratory syncytial virus (RSV)-A2 strain, or adenovirus serotype 3 and analyzed for interleukin (IL)-8 and regulated on activation, normal T-cell expressed and secreted (RANTES) release and mRNA expression. Additionally, activation of nuclear factor (NF)-κB and activator protein (AP)-1 were evaluated. The release of IL-8 and RANTES was also measured in cells stimulated simultaneously with a virus and the HDM allergen, Der f1.
Results
HRV caused greater IL-8 and RANTES release and mRNA expression compared with either RSV or adenovirus. NF-κB and AP-1 were activated in these processes. Cells incubated with a virus and Der f1 showed an increased IL-8 release. However, compared with cells incubated with virus alone as the stimulator, only HRV with Der f1 showed a statistically significant increase.
Conclusions
IL-8 and RANTES were induced to a greater extent by HRV compared with other viruses, and only HRV with Der f1 acted synergistically to induce bronchial epithelial IL-8 release. These findings may correspond with the fact that rhinoviruses are identified more frequently than other viruses in cases of acute exacerbation of asthma.
Respiratory viral infections affect not only asthma development but also exacerbations. Severe viral illnesses in infancy have been linked to an increased risk of asthma development. Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis, and it has recently become evident that human rhinovirus (HRV) infections are the second most common etiology.1 Other viruses that can cause wheezing illnesses include coronaviruses, parainfluenza viruses, metapneumoviruses, and adenoviruses.
Long-term follow-up of infants has showed that HRV-bronchiolitis infections are associated with a greater risk of wheeze compared to RSV-associated bronchiolitis.2 Further, recent epidemiological studies indicate that rhinoviruses are the only type of virus significantly associated with asthma exacerbations in children aged 2-17 years.3 However, it is not entirely understood why rhinoviruses are more frequently associated with asthma development and acute asthma exacerbations compared with other viruses. In addition to respiratory infections, exposure to environmental aeroallergens is an important cause of acute episodes of wheezing. Among aeroallergens, the house dust mite (HDM) allergen Dermatophagoides farinae major allergen 1 (Der f1) is one of the most important contributing factors in asthma exacerbation.
On the basis of these findings, we hypothesized that the mediators from airway epithelial cells elicited by respiratory viruses and Der f1 may differ between rhinoviruses and other respiratory viruses. Among these mediators, IL-8 (CXCL8) is a CXC chemokine with the neutrophil-attractant Glu-Leu-Arg (ERL) motif. Both IL-8 and neutrophils are features of difficult-to-treat asthma phenotypes, similar to virus-induced acute asthma and severe asthma.4,5 Regulated on activation, normal T-cell expressed and secreted (RANTES [CCL5]) is another chemokine that plays an important role in asthma by inducing selective recruitment of Th2-type T-cells and eosinophils. With regard to the transcription of IL-8 and RANTES, previous studies have shown that activation of nuclear factor (NF)-κB or activator protein (AP)-1 can induce the production of these two chemokines, each with distinct kinetics.6-8 To address our question, A549 cells were infected with rhinovirus serotype 7, RSV-A2 strain, and adenovirus serotype 3 with or without Der f1. We analyzed the release and mRNA expression of IL-8 and RANTES. In this process, we also investigated the relationship between the production of chemokines and the activation of NF-κB and AP-1.
We used the A549 cell line, an immortalized line of type II human alveolar epithelial cells derived from a human lung bronchioloalveolar carcinoma. The cells were obtained from the American Type Culture Collection (CCL-185, ATCC). They were cultured in F12 Kaighn's modification (F-12K) media, supplemented with L-glutamine, 10% fetal bovine serum (FBS), and streptomycin/penicillin in a humidified 5% CO2 incubator. All culture materials were purchased from GIBCO (Carlsbad, CA, USA).
Human rhinovirus serotype 7 (VR-1117), RSV A-2 strain (VR-1540), and adenovirus serotype 3 (VR-847) were purchased from ATCC and propagated in cells at 37℃ in a humidified 5% CO2 incubator. HeLa cells were used for rhinovirus; Hep-2 cells, for RSV; and A549 cells, for adenovirus. Briefly, on development of the full cytopathic effect, the cells and supernatants were harvested after three freezing/thawing cycles to rupture the membranes, clarified by centrifugation, aliquoted, and then stored at -70℃.
A549 cells were cultured in 2% FBS media supplemented with F-12K, L-glutamine, 100 µg/mL streptomycin, and 100 U/mL penicillin. The cells were plated in 96-well plates at 1×105 cells/well and cultured overnight at 37℃ in a 5% CO2 incubator. Next, rhinovirus 7, RSV-A2, or adenovirus 3 was added to the cells at 10-1 to 102 of the 50% tissue culture infectious dose (TCID50)/mL and cultured at room temperature for 1 hour with shaking. A549 cells were used for determining the TCID50 in rhinovirus, RSV, and adenovirus. After replacing the media with fresh 2% FBS media supplemented with F-12K (plus L-glutamine, 100 µg/mL streptomycin, and 100 U/mL penicillin), the cells were cultured at 37℃ in a 5% CO2 incubator. The cells were harvested after 24 hours to be assessed by the reverse-transcriptase polymerase chain reaction (RT-PCR). The supernatants were harvested after 1, 3, 6, 12, 24, 36, and 48 hours and stored at -70℃ for until analysis by enzyme-linked immunosorbent assay (ELISA). For experiments using inhibitors of NF-κB and AP-1, cultures were treated with 50 µL pyrrolidine dithiocarbamate (PDTC, Sigma, St. Louis, MO, USA), an NF-κB inhibitor, and 50 µm SP600125C (Sigma), an AP-1 inhibitor, for 24 hours. Next, the levels of IL-8 and RANTES were determined using ELISA.
The IL-8 and RANTES concentrations in the supernatant of the cultured A549 cells were determined using an ELISA kit (BD Biosciences, San Jose, CA, USA), according to the manufacture's protocol. The sensitivity limit of each kit was 10 pg/mL. The assays were performed in duplicate, and mean values are reported.
Total RNA was extracted from cultured A549 cells using the TriZol reagent (Invitrogen, Carlsbad, CA, USA) followed by DNase (Invitrogen) treatment. cDNA was prepared with Superscript reverse transcriptase (Invitrogen). Amplification of IL-8 and RANTES transcripts was accomplished using primers developed according to the published sequence of the human IL-8 and RANTES cDNA. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for verification of equal loading and RT-PCR efficiency. The sequences of the primers (Bioneer, Daejeon, Republic of Korea) were as follows: IL-8, sense 5'-GGACAAGAGCCAGGAAGAAACC-3' and antisense 5'-CTTCAAAAACTTCTCCACAACCC-3'; RANTES, sense 5'-CACTGCCCCGTGCCCACATCAA-3' and antisense 5'-GTAGGCTAATACGACTCACTATAGGGTCCATCTCCA-3'; and GAPDH, sense 5'-ACCACAGTCCATGCCATCAC-3' and antisense 5'-ATGTCGTTGTCCCACCACCT-3'. PCR was performed in a DNA thermal cycler under the following conditions: 25 cycles of denaturation (94℃ for 1 minute), annealing (55℃ for 1 minute), and extension (72℃ for 1 minute), with a final extension for 7 minutes at 72℃. Upon completion, the PCR solution was loaded onto a 1% agarose gel to identify the products.
Nuclear extracts from A549 cells were prepared. Briefly, treated A549 cells were scraped and washed in cold phosphate-buffered saline and resuspended in 500 µL of cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonylfluoride (PMSF) supplemented with proteinase inhibitors). The cells, swollen at 4℃ for 10 minutes, were lysed and the homogenate centrifuged for 5 minutes at 600×g. The nuclear pellet was resuspended in 30 µL of cold buffer C (20 mM HEPES [pH 7.9], 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, and 0.5 mM PMSF) supplemented with proteinase inhibitors and stored in small aliquots at -70℃.
Prepared protein from the nuclear extract was analyzed using SDS-PAGE and western blotting with polyclonal antibodies specific for phosphorylated forms of p65 NF-κB subunits (Santa Cruz Biotechnology, Inc.). The intensities of the bands on the immunoblot were quantified using densitometry.
Experiments were treated with 50 µM pyrollidine dithiocarbamate (PDTC, Sigma, St. Louis, MO, USA), which is an NF-κB inhibitor, and 50 µM SP600125C (Sigma), which is an AP-1 inhibitor, for 24 hours. Next, the levels of IL-8 and RANTES were determined using ELISA.
To simulate exposure to natural Der f1 (Indoor Biotechnologies, Warminster, UK), the antigen was added to cultured A549 cells at 1 µg/mL and incubated at 37℃ in a 5% CO2 incubator for 24 hours. For combined viral and Der f1 exposure, Der f1 was added to cultured A549 cells at 1 µg/mL and cultured at 37℃ in a 5% CO2 incubator for 6 hours. Following the addition of fresh media, the cells were infected with rhinovirus, RSV, or adenovirus at 102 TCID50/mL, and shaken at room temperature for 1 hour, replenished with fresh media, and cultivated at 37℃ in a 5% CO2 incubator for an additional 18 hours.
Data are expressed as a mean (standard deviation). Statistical analyses were performed using the SPSS software package (ver. 12.0 for Windows; SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used to compare the mean values. P values of <0.05 were deemed to indicate statistical significance.
Significant IL-8 release was induced by rhinovirus, RSV, and adenovirus (P<0.05), and an increased amount of IL-8 was released in response to rhinovirus compared to RSV or adenovirus (P<0.05). IL-8 release did not differ significantly in response to RSV and adenovirus infection (Fig. 1A). All three viruses induced IL-8 mRNA expression 24 hours after viral infection. IL-8 mRNA expression was increased in a dose-dependent fashion. A modest increase in IL-8 mRNA was detected even at 10-1 TCID50/mL of rhinovirus, but not RSV or adenovirus (Fig. 1B).
Rhinovirus and RSV induced a significant increase in the release of RANTES (P<0.05). However, the release of RANTES did not increase significantly after stimulation with adenovirus. Rhinovirus induced RANTES production to a greater extent than RSV (P<0.05; Fig. 2A). At 24 hours after viral infection, all three viruses induced RANTES mRNA expression in a dose-dependent fashion. Only rhinovirus showed RANTES mRNA expression even at 10-1 TCID50/mL of viral infection (Fig. 2B).
We examined the effect of rhinovirus, RSV, and adenovirus on NF-κB activation. Phosphorylated forms of p65 NF-κB subunits increased when cultured A549 cells were infected with 102 TCID50/mL of rhinovirus, RSV, or adenovirus. Relative pp65 expression was calculated as the pp65/β-actin ratio from the control. The activation of NF-κB was induced more efficiently by rhinovirus and RSV compared to adenovirus (Fig. 3). The effect of the transcription factor inhibitor was assessed by comparing the release of IL-8 and RANTES from A549 cells after exposure to each virus. The NF-κB inhibitor decreased the release of IL-8 and RANTES by each of the 3 viruses. (P<0.05; Fig. 4A and B).
In addition to NF-κB, we also analyzed the production of the phosphorylated subunit of AP-1 (p-c-Jun) using SDS-PAGE. All three of the viruses induced the activation of AP-1. However, the viruses had different time courses: AP-1 was activated rapidly by rhinovirus, then by adenovirus and finally by RSV (Fig. 4). AP-1 inhibitor also suppressed the release of IL-8 and RANTES from A549 cells after exposure to each virus (P<0.05; Fig. 4A and B).
Der f1 induced the release of IL-8 from A549 cells. When the cells were stimulated with both virus and Der f1, an increase in IL-8 release was observed only in response to rhinovirus (P<0.05; Fig. 5). IL-8 release from cells stimulated with RSV plus Der f1 or adenovirus plus Der f1 was not statistically different from the cells stimulated with virus alone (Fig. 5). Der f1 did not induce the release of RANTES (data not shown).
We compared three different respiratory viruses in terms of chemokine expression by infecting human respiratory epithelial cells. HRV caused a greater release of IL-8 and RANTES than RSV or adenovirus. Thus, we can confirm that the activation of the central transcription step, the NF-κB and AP-1 pathway, was involved in these processes. We also showed that the combined stimuli of rhinovirus and Der f1 induced IL-8 release to a greater extent than rhinovirus alone.
Chemokines mediate cell recruitment to inflammation sites and influence the production of various cytokines. We primarily focused on the measurement of IL-8/CXCL8 because it is one of the major chemokines that can be produced in large amounts from airway epithelium, in response to several stimuli including proinflammatory cytokines, viruses, microbes, allergens, and other environmental factors.9 IL-8 acts as a key mediator in neutrophil-mediated acute inflammation.10 Neutrophil activation is believed to contribute to airway obstruction and lower airway symptoms following respiratory viral infection. An increase in neutrophils has been observed in the lower airways of infants with recurrent wheezing.11 Increased airway neutrophils and IL-8 production are found in patients with severe asthma,5 as well as acute exacerbations of asthma induced by HRV.4 HRV, RSV, and adenovirus examined in the present study can cause wheezing illnesses. Our results showed that these viruses could induce IL-8 release.
While neutrophils are considered to play an important role in intractable and severe asthma,5 eosinophils remain the characteristic cells of asthma. The CC chemokines eotaxin, RANTES, and MIP-1α are known to be potent chemoattractants for eosinophils. In particular, RANTES is another key proinflammatory chemokine produced by virus-infected epithelial cells and is present in the respiratory secretions of asthmatics.12 Compared with respiratory disease-free children, greater concentrations of RANTES and MIP-1α have been demonstrated in nasopharyngeal secretions and tracheal aspirates of infants with RSV-associated bronchiolitis.13 RANTES not only plays a major role in governing eosinophilia but also in controlling viral replication by recruiting CD4+ and CD8+ T cells.11 In our study, rhinovirus and RSV showed statistically significant increases in the release of RANTES, but this was not the case for adenovirus. Many other reports show the relationship between rhinovirus or RSV and RANTES secretion in airway epithelial cells. Konno et al.14 reported that the secretion of IFN-γ by T-cells and NK-cells within airway tissues during the early stages of rhinovirus infection could regulate interactions between viral particles and host epithelial cells, and subsequent RANTES secretion. They suggested that because RANTES is a potent chemoattractant for memory T-cells, monocytes, eosinophils, and basophils, augmentation of RANTES secretion in vivo could enhance airway inflammation and promote airway obstruction. John et al.15 showed that RSV-induced RANTES contributes to exacerbation of allergic airway inflammation in a study utilizing a murine model. However, in the case of adenovirus infection, there are no previous reports that analyze the relationship between adenovirus and RANTES secretion in acute asthma exacerbation. The present study did not find a correlation between RANTES and adenovirus in the airway epithelial cells.
Most HRV serotypes and RSV strains cause little cellular injury to cultured bronchial epithelial cells compared to infections with adenovirus.16,17 The majority of HRV- and RSV-induced pathology is attributed to indirect stimulation of host innate and adaptive immune cells. Bronchoalveolar lavage (BAL) fluid recovered from minor-group RV1B HRV-infected mice demonstrates neutrophilia, upregulation of interleukin (IL)-6, IL-1b, RANTES, and macrophage inflammatory protein-2 (MIP-2) within the first 2 days after infection.3,17,18 In a human study, Gern et al.19,20 also observed elevated secretion of the neutrophil chemotaxin IL-8, granulocyte colony-stimulating factor (G-CSF), IP-10, and MIP-2 in the sputum samples of rhinovirus-infected asthma patients. Neutrophil recruitment was correlated with virus-mediated influx of IL-8 in both asthmatic and non-atopic individuals.4 In the case of RSV infection, neutrophils are also abundant in the nasal lavages of RSV-infected adults and infants,21 and early cytokines and chemokines such as IL-12, IL-18, MIP-1a, IL-8, and RANTES are upregulated in the upper respiratory tract.22 These studies provide evidence that the infiltrating cell and chemokine profiles of both viruses are similar in virus-infected murine models and human subjects.
In the present study, among the A549 cells infected with rhinovirus, RSV, or adenovirus, the release of IL-8 and RANTES was greatest in cells infected with rhinovirus. Although, numerous studies in vitro have demonstrated that rhinovirus is a more potent inducer of chemokine production in infected airway epithelial cells, this is the first study that has shown the superiority of rhinovirus in a direct comparison with other respiratory viruses. Considering the roles of IL-8 and RANTES in the acute exacerbation of asthma, we suggest that the higher release of these chemokines from airway epithelial cells after rhinovirus infection might be associated with the fact that rhinovirus is the major cause of acute exacerbations of asthma.
NF-κB is a transcription factor, implicated in the expression of over 100 proinflammatory genes that mostly participate in the host immune response.23 Conclusive evidence suggests that rhinovirus-induced inflammation is caused by NF-κB activation.24 A significant volume of research has been made to prove that NF-κB inhibitors constitute a potential therapeutic treatment for rhinovirus-induced asthma exacerbation. In the present study, all three viruses activated the NF-κB pathway, and the use of a NF-κB inhibitor suppressed virus-induced IL-8 and RANTES production. Van Ly et al.23 recently showed that the NF-κB inhibitor BAY-117085 (BAY) inhibited rhinovirus-induced IL-6, but not IL-8. They suggested that NF-κB activation alone may not be the causative factor for rhinovirus-induced IL-8 production, but perhaps the combined activation of NF-κB, AP-1, and CCAAT/enhancer binding protein (C/EBP) may be more important. However, in the present study, we used PDTC as an inhibitor of NF-κB and successfully suppressed the production of IL-8 and RANTES by viral infection. In addition, activation of AP-1 with all three viruses was observed, and an inhibitor of AP-1 also suppressed virus-induced chemokine production. These findings can be interpreted as NF-κB and AP-1 pathways being involved in virus induced IL-8 and RANTES production, each with distinct kinetics. To develop an effective therapeutic strategy for respiratory virus induced asthma exacerbation, combined treatments incorporating an NF-κB inhibitor, multiple NF-κB inhibitors, or inhibitors of other transcription factors such as AP-1 may be effective.
Finally, this study yielded new data relating to the inflammatory response of human alveolar epithelial cells after exposure to two major environmental stimuli associated with asthma exacerbation. We showed that rhinovirus and Der f1 are synergistic with regard to the induction of IL-8, but this synergy was not observed in the case of RSV or adenovirus. Bossios et al.25 described the stimulation of IL-8 release from BEAS-2B cells by the HDM Dermatophagoides pteronyssinus major allergen 1 (Der p 1), HRVs (HRV1b or HRV16), or both in different sequences. In these experiments, both rhinovirus and Der p 1 induced IL-8, IL-6, IL-29, and IP-10, while RANTES was induced only by rhinovirus. Further, synergistic induction was observed only for IL-8, giving similar results to the results presented in this study. In our study Der f1 and three respiratory viruses induced IL-8, while RANTES was not induced by Der f1. Further, viruses with Der f1 were not synergistic with regard to the induction of RANTES. However, a previous study reported that synergy was not observed when cells were initially exposed to Der p 1 and then to HRV.25 In contrast, our study showed that exposure to an allergen, specifically Der f1, preceding viral infection yielded a significant increase in IL-8 production. Recently a birth cohort study showed that allergic sensitization precedes HRV wheezing and that the reverse is not true.26 This sequential relationship, whereby allergic sensitization can lead to more severe HRV-induced lower respiratory illnesses is consistent with our findings. Furthermore, this cohort study showed that allergic sensitization led to an increased risk of wheezing illnesses caused by HRV, but not RSV.
In contrast to the present study where Der f1 and RSV did not show synergistic induction, a synergistic effect between Der p 1 and RSV, during the induction of IL-8 by epithelial cells, has been reported.27 This may reflect differences in the infectious dose of RSV used or in the exposure time of Der f1 allergen. Because our study was mainly focused on the comparison of three different respiratory viruses under the same conditions, the amount of viral infection had to be standardized. Considering dust mite allergen action is short lived, showing its effect during the first few hours of exposure,28 the exposure time to Der f1 in the present study is thought to be sufficient. As a result, when all conditions are equal, the rhinovirus is the only virus that can act synergistically with Der f1 in the induction of IL-8. Such a characteristic of rhinovirus may represent an important mechanism in virus-induced asthma exacerbations.
Although the use of A549 cells derived from a human alveolar cell carcinoma with properties of type II alveolar epithelial cells allow for the detailed examination of respiratory virus/epithelial cell interactions to the same extent as BEAS-2B, 16HBE and HEp-2 cells,29,30 a potential limitation of these studies is that the isolated epithelial cells are not sufficient to explain the systemic immune response that occurs in real airways. In the present study, we tested doses and time-points found in the literature25,31,32 and confirmed these further in our preliminary experiments. However, we cannot exclude the possibility that other doses and time points could modify our results.
The presented study is distinguished from previous studies in that we compared the effect of three different respiratory viruses on airway epithelial cells under identical conditions. We observed for the first time that rhinovirus is superior to RSV or adenovirus in inducing the production of IL-8 and RANTES. In this process, we investigated possible molecular mechanisms leading to these results by measuring the central transcription step, NF-κB, and AP-1 translocation. We also showed that HRV and Der f1, two major environmental stimuli, operate synergistically in the induction of IL-8. This phenomenon may be involved in the pathogenesis of viral-induced acute exacerbations of asthma, as rhinovirus is the virus most frequently associated with asthma exacerbations.
Figures and Tables
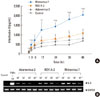 | Fig. 1(A) Effects of viral infection on IL-8 release (mean [standard deviation]) from A549 cells, as assessed by ELISA. The increase in IL-8 release was as follows: rhinovirus>respiratory syncytial virus (RSV)=adenovirus>control. A549 cells were infected at 102 50% tissue culture infectious dose (TCID50)/mL (n=3). *P<0.05, compared with the control, †P<0.05, compared with adenovirus, ‡P<0.05, compared with RSV. (B) IL-8 mRNA expression in A549 cells infected with rhinovirus, RSV, or adenovirus at 10-1 to 102 TCID50/mL. The mRNA expression of IL-8 occurred in decreasing order at 24 hr after infection with rhinovirus, RSV, and adenovirus. TNF α (10 ng/mL) and IL-4 (10 ng/mL) were stimulators for the positive control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. |
 | Fig. 2(A) Effects of viral infection on RANTES release (mean [standard deviation]) from A549 cells, as assessed by ELISA. The increase in RANTES release was as follows: rhinovirus>RSV>adenovirus=control. A549 cells were infected at 102 TCID50/mL (n=3). *P<0.05, compared with the control, †P<0.05, compared with adenovirus, ‡P<0.05, compared with RSV. (B) RANTES mRNA expression in A549 cells infected with rhinovirus, RSV, or adenovirus at 10-1 to 102 TCID50/mL. The mRNA expression of RANTES occurred in decreasing order at 24 hr after infection with rhinovirus, RSV, and adenovirus. TNF-α (10 ng/mL) and IL-4 (10 ng/mL) were stimulators for the positive control. GAPHD was used as a housekeeping gene. |
 | Fig. 3(A) Phosphorylated subunits of NF-κB (pp65) were analyzed by SDS-PAGE. The band densities were determined by densitometry. Relative pp65 expressions were calculated as the pp65/β-actin ratio of control. (B) Effect of NF-κB inhibitor on the release of IL-8 and RANTES from A549 cells after 48 hr infection assessed by ELISA. All three viruses induced IL-8 and RANTES release compared with the control medium (*P<0.05). Fifty µM pyrollidine dithiocarbamate (PDTC) suppressed the release of IL-8 and RANTES in all three viruses compared with virus only (†P<0.05). Data are from three independent experiments and results are presented as the mean±standard deviation. |
 | Fig. 4(A) Phosphorylated subunits of AP-1 (p-c-Jun) were analyzed by SDS-PAGE. The band densities were determined by densitometry. Relative p-c-Jun expressions were calculated as the p-c-Jun/β-actin ratio of control. (B) Effect of AP-1 inhibitor on the release of IL-8 and RANTES from A549 cells 48 hr after infection assessed by ELISA. All three viruses induced increased IL-8 and RANTES release compared with the control medium (*P<0.05). Fifty µM SP600125C suppressed induction of IL-8 and RANTES by all three viruses compared with virus only (†P<0.05). Data are from three independent experiments and results are presented as mean±standard deviation. |
 | Fig. 5The release of IL-8 (mean [standard deviation]) from A549 cells after viral infection with or without exposure to dust mite allergen Der f1 (1 µg/mL) as assessed by ELISA. All stimuli induced an increase in IL-8 release compared with the control medium at 24 hr after infection. Only rhinovirus with Der f1 induced a greater increase in IL-8 release compared with single viral stimulation. Each virus was infected with 102 TCID50/mL (n=3). *P<0.05, compared with the control, †P<0.05, virus alone versus in combination with Der f1. |
ACKNOWLEDGMENTS
This work was partly supported by a Korea Research Foundation (KRF) grant, funded by the Korean Government (MEST) (No. 2009-0066649) and by Seoul St. Mary's Clinical Medicine Research Program of 2009 through the Catholic University of Korea. The authors would like to thank the Vaccine Biomedical Engineering Institute of the Catholic University of Korea for its support during this study.
References
1. Kim WK, Gern JE. Updates in the relationship between human rhinovirus and asthma. Allergy Asthma Immunol Res. 2012; 4:116–121.
2. Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003; 111:66–71.
3. Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010; 125:1178–1187. quiz 1188-9.
4. Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002; 19:68–75.
5. Wenzel S. Mechanisms of severe asthma. Clin Exp Allergy. 2003; 33:1622–1628.
6. Ammit AJ, Lazaar AL, Irani C, O'Neill GM, Gordon ND, Amrani Y, Penn RB, Panettieri RA Jr. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002; 26:465–474.
7. Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000; 275:18138–18144.
8. Thomas LH, Wickremasinghe MI, Friedland JS. IL-1 beta stimulates divergent upper and lower airway epithelial cell CCL5 secretion. Clin Immunol. 2007; 122:229–238.
9. Strieter RM. Interleukin-8: a very important chemokine of the human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L688–L689.
10. Sehmi R, Cromwell O, Wardlaw AJ, Moqbel R, Kay AB. Interleukin-8 is a chemo-attractant for eosinophils purified from subjects with a blood eosinophilia but not from normal healthy subjects. Clin Exp Allergy. 1993; 23:1027–1036.
11. Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, Wenzel SE. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001; 163:1338–1343.
12. Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, Hussell T, Openshaw PJ. Role of CCL5 (RANTES) in viral lung disease. J Virol. 2006; 80:8151–8157.
13. Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sánchez PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999; 18:115–122.
14. Konno S, Grindle KA, Lee WM, Schroth MK, Mosser AG, Brockman-Schneider RA, Busse WW, Gern JE. Interferon-gamma enhances rhinovirus-induced RANTES secretion by airway epithelial cells. Am J Respir Cell Mol Biol. 2002; 26:594–601.
15. John AE, Berlin AA, Lukacs NW. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur J Immunol. 2003; 33:1677–1685.
16. Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002; 76:5654–5666.
17. Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, Gern JE. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999; 20:1220–1228.
18. Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffery PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blair ED, Clarke NJ, Johnston SL. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008; 14:199–204.
19. Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000; 162:2226–2231.
20. Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009; 9:73–78.
21. Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004; 189:1419–1430.
22. Wong T, Hellermann G, Mohapatra S. The infectious march: the complex interaction between microbes and the immune system in asthma. Immunol Allergy Clin North Am. 2010; 30:453–480.
23. Van Ly D, King NJ, Moir LM, Burgess JK, Black JL, Oliver BG. Effects of beta(2) Agonists, Corticosteroids, and Novel Therapies on Rhinovirus-Induced Cytokine Release and Rhinovirus Replication in Primary Airway Fibroblasts. J Allergy (Cairo). 2011; 2011:457169.
24. Edwards MR, Hewson CA, Laza-Stanca V, Lau HT, Mukaida N, Hershenson MB, Johnston SL. Protein kinase R, IkappaB kinase-beta and NF-kappaB are required for human rhinovirus induced pro-inflammatory cytokine production in bronchial epithelial cells. Mol Immunol. 2007; 44:1587–1597.
25. Bossios A, Gourgiotis D, Skevaki CL, Saxoni-Papageorgiou P, Lötvall J, Psarras S, Karpathios T, Constandopoulos AG, Johnston SL, Papadopoulos NG. Rhinovirus infection and house dust mite exposure synergize in inducing bronchial epithelial cell interleukin-8 release. Clin Exp Allergy. 2008; 38:1615–1626.
26. Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, Gern JE, Lemanske RF Jr. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012; 185:281–285.
27. Foster S, Bedford KJ, Gould ME, Coward WR, Hewitt CR. Respiratory syncytial virus infection and virus-induced inflammation are modified by contaminants of indoor air. Immunology. 2003; 108:109–115.
28. Grunstein MM, Veler H, Shan X, Larson J, Grunstein JS, Chuang S. Proasthmatic effects and mechanisms of action of the dust mite allergen, Der p 1, in airway smooth muscle. J Allergy Clin Immunol. 2005; 116:94–101.
29. Xatzipsalti M, Papadopoulos NG. Cellular and animals models for rhinovirus infection in asthma. Contrib Microbiol. 2007; 14:33–41.
30. Thomas LH, Friedland JS, Sharland M, Becker S. Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on nuclear factor-kappa B nuclear binding and is inhibited by adenovirus-mediated expression of inhibitor of kappa B alpha. J Immunol. 1998; 161:1007–1016.
31. Yoon JS, Kim HH, Lee Y, Lee JS. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epithelial cells. Pediatr Pulmonol. 2007; 42:277–282.
32. Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006; 281:6910–6923.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download