Abstract
Purpose
Filaggrin (FLG) is a key protein that facilitates the terminal differentiation of the epidermis and the formation of the skin barrier. Recent studies showed that atopic dermatitis (AD) associates closely with loss-of-function mutations in the FLG gene. Asian and European populations differ in the frequencies of FLG mutations. Several FLG mutations, including 3321delA, E2422X, K4671X, S2554X, and R501X, occur frequently in Chinese and Japanese populations. The association between three FLG null mutations and AD in Korean children was investigated.
Methods
The FLG mutations in 1,430 children (aged 0-18 years) with AD and 862 control subjects were genotyped by using the TaqMan assay.
Results
The FLG null mutation E2422X was not detected in any patients with AD or control subjects. The R501X null mutation was detected in only one child with AD (0.1%). Children with AD had the 3321delA deletion significantly more frequently (2.4%) than the control subjects (0.0%, P<0.001). Children with AD also had a significantly higher combined allele frequency of the three FLG null mutations (2.6%) than the controls (0.0%, P<0.001). The 3321delA null mutation did not associate significantly with AD severity (P=0.842). When the patients with AD were divided into allergic AD and non-allergic AD patient groups, these two groups did not differ in terms of the frequency of 3321delA.
Atopic dermatitis (AD) is a chronic relapsing skin disease that is frequently associated with other atopic conditions such as asthma and allergic rhinitis.1 The skin of AD patients is characterized by immune dysregulation and epidermal barrier defects such as abnormal terminal differentiation of keratinocytes and decreased cornification.2-4 Recent studies showed that these characteristics of AD associate closely with loss-of-function mutations (null) in the filaggrin (FLG) gene. FLG deficiency has been shown experimentally to lead to a failure in the barrier function of the skin in humans.5,6
In mice, loss-of-function FLG mutations result in the absence or reduction of the FLG protein and lead to a compromised skin barrier that allows the entry of allergens, which then trigger an immunological response.7 Several studies also show that FLG null mutations associate significantly with AD. In 2006, Palmer et al.8 reported that the R501X and 2282del4 nonsense mutations were associated with a highly significant dominant risk of AD in many European populations. A study by Ching et al.10 also found that R501X was present in 2.3% of Chinese patients with AD but absent in the controls. Hamada et al.11 found R501X in and two Japanese individuals with severe ichthyosis vulgaris (IV). The E2422X mutation, which was previously found in a single Dutch patient,9 was also detected in one of 100 Singaporean patients with IV12 and two Chinese families with IV, but not in controls.13
The 3321delA mutation appears frequently in Asian populations. It was first reported by a study on 143 Japanese patients with AD: 1.4% of the AD cohort had this mutation while it was absent in Japanese nonatopic and nonichthyotic controls.14 Also, this mutation was reported in Asian including Korean patients with IV.13,15,16 In 2011, Zhang et al.17 showed that the 3321delA was associated with AD and asthma compared to control. However, this mutation was not found in a European population,8 which suggests that this mutation may be more common in the Asian populations.
On the basis of these observations, the present study examined the association between the three FLG mutations 3321delA, R501X, and E2422X and susceptibility to and severity of AD in Korean children.
In total, 2,292 children (0-18 years) were enrolled, namely 1,430 patients with AD and 862 normal control subjects who lacked a history of AD or asthma or any atopic manifestations. The subjects with AD were examined at a childhood Asthma Atopy Center, Asan Medical Center in Seoul and diagnosed according to the criteria of Hanifin and Rajka.18 The subjects with AD were divided into two groups: those with positive skin prick test results and specific IgE levels (the allergic AD group) and those with negative results (the non-allergic AD group). AD disease severity was assessed by using the SCORing Atopic Dermatitis index (SCORAD) and the patients with AD were grouped into mild (<15 points), moderate (15-40 points) or severe (>40 points) disease groups. The human ethics committee of the Asan Medical Center Institutional Review Board approved this study. Written informed consent was obtained from the parents of all subjects.
The patients were tested for the 27 common aeroallergens by using the skin prick test. These included the house dust mites Dermatophagoides pteronyssinus, D. farinae, cat, dog, cockroach, the molds Alternaria alternata and Aspergillus fumigatus, grass, tree mix (I), tree mix (II), poak, mugwort, ragweed, egg, and milk etc.19 The allergen-induced wheal that developed after pricking was considered to be positive if it was bigger than the histamine-induced wheal, which was bigger than 3 mm in diameter. Peripheral blood samples were collected from the patients with AD and controls and analyzed for total serum IgE levels and specific IgE levels, including IgE specific for inhalant mix (Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog and cockroach) and food mix (egg, milk, soybean, peanut, fish and wheat). For this, a fluorescent enzyme immunoassay was used (AutoCAP system, Pharmacia Diagnostics AB, Uppsala, Sweden). Antigen-specific IgE concentrations ≥0.35 KIU/L were considered to be positive.
Genomic DNA samples were extracted from peripheral whole blood by using the Gentra Puregene Blood kit (QIAGEN Biotech, Seoul, Korea). Genotyping for the FLG mutations 3321delA, E2422X and R501X was conducted by using the TaqMan assay (ABI, Foster City, CA, USA) with a 384-well plate. The final volume of each polymerase chain reaction (PCR) mixture was 5 µL and it contained 10 ng of genomic DNA, 2.5 µL TaqMan Universal PCR Master Mix, and 0.26 µL of 40X Assay Mix.
Statistic analyses were performed by using SPSS version 19. The continuous clinical characteristic variables were expressed as mean±standard deviations (SDs) and differences between groups in terms of these variables were tested by using independent t-test. Chi-square test was used to examine differences between groups in terms of categorical variables and to compare the allelic and genotype frequencies. For all analyses, a P value <0.05 was regarded as being statistically significant.
The clinical characteristics of the patients with AD and the controls are presented in Table 1. The patients with AD were 5.17 years old on average and 57.3% were male. The controls were 9.47 years old on average and 49.7% were male. When AD severity was determined by generating objective SCORAD scores, 809, 383, and 210 of the patients with AD were found to have mild, moderate and severe disease, respectively.
The patients and controls were genotyped for the three previously reported null mutations 3321delA, R501X, and E2422X, and the association between these mutations and susceptibility to AD was analyzed. The 3321delA deletion was detected in 28 (2.4%) patients with AD but none of the control subjects (P<0.001). Neither E2422X nor R501X were associated with susceptibility to AD: none of the patients with AD and the control subjects had E2422X while only one subject with AD (0.1%) had null mutation of R501X (P=0.446; Table 2). Analysis of the combination of the three null mutations revealed that it associated highly significantly with susceptibility to AD (P<0.001; Table 3)
No significant associations were detected between AD severity and the 3321delA mutation (P=0.842; Table 4). While both the non-allergic AD and allergic AD groups had the 3321delA mutation significantly more frequently than the control group (P<0.001 for both; Table 5), the non-allergic AD and allergic AD groups did not differ in terms of the frequency of this mutation.
In the present study, the 3321delA associated significantly with the development of AD. This confirms previous studies that FLG null mutations are associated with AD.10,14,17,20-22 The primary purpose of these study was to identify associations between the FLG mutations R501X, E2422X and 3321delA and susceptibility to and severity of AD in Korean children. In result, we found a significantly higher frequency of the 3321delA and combination of three mutations in AD patients compared to controls. However, we did not find significant association between severity of AD and occurrence of FLG mutation. This finding suggests that FLG mutations may contribute to the genetic susceptibility to AD in Korea as well as in other countries. However, the frequencies of the R501X and E2422X in Koreans were lower than that in Europeans.
Impairment of epidermal barrier function is a clinical hallmark of AD.2-4 FLG is thought to be one of the most important factors in skin barrier function. Mildner et al.5 demonstrated that knockdown of FLG expression in human skin model reproduced the epidermal alterations caused by FLG mutations. Moreover, lack of FLG led to a reduction in the concentration of urocanic acid, which is released by proteolysis of FLG.5 In experimental, immunohistochemical staining for filaggrin revealed that mutation leads to remarkable reduction of filaggrin protein expression in the AD patients' epidermis.23 And the clinical severity significantly correlated with transepidermal water loss, stratum corneum hydration and thickness in filaggrin related AD.24 These findings suggest that FLG dysfunction is an important in the development of AD.
In the last few years, FLG mutations have been identified widely in European, American, and Asian. The previously reported FLG mutations seem to be population-specific: in particular, R501X is common in Europeans9 but is rarely found in Chinese10,17 and Japanese people.11,20 With regard to E2422X and 3321delA were mainly founded in Asian patients with AD.21,22 Thus, three null mutations R501X, E2422X and 3321delA were selected for analysis in the present study. The 3321delA deletion was detected in 28 AD patients (2.4%), but not in controls; and, E2422X and R501X did not associate significantly with susceptibility to AD. The combination of the three mutations was present in 29 (2.6%) patients with AD and none of the healthy controls, indicating a strong association between these FLG mutations and AD (P<0.001; Table 3). These observations indicate that FLG mutations are significant predisposing factors for AD in Koreans.
Overall, the participants in the present study had a low frequency of FLG mutations (ranging from 0% to 2.6%). Similarly, in Chinese patients with AD, only 2.3% and 0.3% had the R501X10 and E2422X22 mutations, respectively. Indeed, the most common mutation in Asian populations is 3321delA, yet it is present in only 9.7% of Chinese patients with AD22 and 1.4% of Japanese patients with AD.14 These results indicate that the frequency of FLG mutations is lower in Asians, including in Korean children, than in Europeans.
The present study also investigated the relationship between FLG mutations and atopy by measuring the frequency of the 3321delA mutation in patients with allergic AD and non-allergic AD. However, the two groups did not differ significantly. This suggests that FLG mutations may have two pathogenic effects. First, FLG mutations may directly affect the structure of the skin itself, thereby reducing its ability to act as a barrier. Supporting this are the associations between FLG mutations and IV in Chinese12,13 and Japanese populations.11 Second, FLG mutation is functionally related to the skin barrier and therefore is most likely to be associated with the cause of skin disease including AD. However, FLG may also affect other allergic disease such as asthma. Supporting this is that FLG variants increase the risk of atopic asthma in the absence of AD or a history thereof,25 and asthma without eczema.26 Also, Chinese patients with AD were interested significant association between FLG mutation and AD with asthma.17 These associations between FLG mutations and allergic disease have been explained by the outside-inside hypothesis.27 It proposes that a barrier abnormality is the driving force behind the development of an inflammatory response in patients with AD. They subsequently hypothesized that reduced levels of FLG, in particular its acidic derivative urocanic acid, increase the pH of the stratum corneum, thereby altering the activity of the multiple serine proteases and two ceramide-generating enzymes that regulate the homeostasis of the stratum corneum.3 Another important downstream consequence of the increased pH and serine protease activity is the generation of active primary cytokines such as IL-1α, IL-1β, IL-25, and IL-33 from their inactive proproteins.28,29 Moreover, defective barrier that leads to a Th2-dominant infiltrate is proposed to be a secondary cause of the inflammation by antigens seen in patients with AD.29 In the present study, FLG mutations were not associated with the severity of AD. Two studies were reported that there was no association between FLG mutation and the severity of AD in Chinese17 and Japanese24 like out results, on the other hand there was a significant association in Singaporean21 and Chinese.10 Also there were several studies that FLG mutations affect to the severity of AD in European.30,31 From these results, the severity of AD may be influenced by FLG mutation in many populations but it seems not to be specific to ethnic. Taken together, FLG is not only a major gene for the development of AD, but that it may also participate in susceptibility to allergic disease. The occurrence of AD in lacking FLG null mutations may be caused by variants in genes pathway that modify filaggrin protein processing or unknown mutation of other independent genes in the skin barrier including stratum corneum and tight junction through environment variations. It is expected that follow-up genetic studies will uncover further genetic loci important in AD.
The present study had several limitations. First, rather than fully sequencing FLG to identify target mutations, three known mutations were examined. Therefore, the effect of other mutations on the development of or severity of AD could not be determined. Second, the present study could not show whether FLG mutation actually causes skin barrier dysfunction. Therefore, additional experiments should be performed to elucidate how FLG mutations promote the development of AD.
In conclusion, FLG null mutations significantly associated with the development of AD in Korean children, although the frequencies of the FLG mutations that were examined were low overall. Mechanism between FLG mutations and Th2 response through defects of skin barrier should be examined for new therapeutic target of AD.
Figures and Tables
Table 1
Characteristics of the study subjects
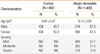
Table 2
Frequencies of three filaggrin (FLG) mutations in atopic dermatitis and control subjects

Table 3
Frequencies of the combination of the filaggrin (FLG)-null mutations 3321delA, E2422X and R501X in atopic dermatitis and control subjects

| FLG | Control N (%) | Atopic dermatitis, N (%) | P value |
|---|---|---|---|
| Normal | 704 (100.0) | 1,099 (97.4) | |
| Any one | 0 (0.0) | 29 (2.6) | < 0.001 |
Table 4
Association between the filaggrin mutation 3321delA and the severity of atopic dermatitis

| 3321delA | Mild N (%) | Moderate N (%) | Severe N (%) | P value |
|---|---|---|---|---|
| Normal | 603 (97.6) | 280 (97.9) | 228 (97.0) | 0.842 |
| Null | 15 (2.4) | 6 (2.1) | 7 (3.0) |
Table 5
Association between the filaggrin mutation 3321delA and non-allergic and allergic atopic dermatitis patients relative to the control group

| 3321delA | Control N (%) | Non-allergic AD N (%) | P value | Allergic AD N (%) | P value |
|---|---|---|---|---|---|
| Normal | 759 (100.0) | 288 (97.4) | 833 (97.4) | ||
| Null | 0 (0.0) | 6 (2.6) | < 0.001 | 22 (2.6) | < 0.001 |
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health, Welfare, Republic of Korea (A092076).
References
1. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003; 112:S118–S127.
2. Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, Cardinale I, Lin P, Bergman R, Bowcock AM, Krueger JG. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009; 124:1235–1244.e58.
3. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009; 9:437–446.
4. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008; 121:1337–1343.
5. Mildner M, Jin J, Eckhart L, Kezic S, Gruber F, Barresi C, Stremnitzer C, Buchberger M, Mlitz V, Ballaun C, Sterniczky B, Födinger D, Tschachler E. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010; 130:2286–2294.
6. Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, Presland RB, Fleckman P, Janecke AR, Sandilands A, McLean WH, Fritsch PO, Mildner M, Tschachler E, Schmuth M. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011; 178:2252–2263.
7. Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, Callanan JJ, Kawasaki H, Shiohama A, Kubo A, Sundberg JP, Presland RB, Fleckman P, Shimizu N, Kudoh J, Irvine AD, Amagai M, McLean WH. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009; 41:602–608.
8. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446.
9. Sandilands A, Terron-Kwiatkowski A, Hull PR, O'Regan GM, Clayton TH, Watson RM, Carrick T, Evans AT, Liao H, Zhao Y, Campbell LE, Schmuth M, Gruber R, Janecke AR, Elias PM, van Steensel MA, Nagtzaam I, van Geel M, Steijlen PM, Munro CS, Bradley DG, Palmer CN, Smith FJ, McLean WH, Irvine AD. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007; 39:650–654.
10. Ching GK, Hon KL, Ng PC, Leung TF. Filaggrin null mutations in childhood atopic dermatitis among the Chinese. Int J Immunogenet. 2009; 36:251–254.
11. Hamada T, Sandilands A, Fukuda S, Sakaguchi S, Ohyama B, Yasumoto S, McLean WH, Hashimoto T. De novo occurrence of the filaggrin mutation p.R501X with prevalent mutation c.3321delA in a Japanese family with ichthyosis vulgaris complicated by atopic dermatitis. J Invest Dermatol. 2008; 128:1323–1325.
12. Chen H, Ho JC, Sandilands A, Chan YC, Giam YC, Evans AT, Lane EB, McLean WH. Unique and recurrent mutations in the filaggrin gene in Singaporean Chinese patients with ichthyosis vulgaris. J Invest Dermatol. 2008; 128:1669–1675.
13. Zhang X, Liu S, Chen X, Zhou B, Liu D, Lei G, Xiao X, Liu H, Wang H. Novel and recurrent mutations in the filaggrin gene in Chinese patients with ichthyosis vulgaris. Br J Dermatol. 2010; 163:63–69.
14. Nomura T, Sandilands A, Akiyama M, Liao H, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H, Palmer CN, Smith FJ, McLean WH, Shimizu H. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol. 2007; 119:434–440.
15. Hsu CK, Akiyama M, Nemoto-Hasebe I, Nomura T, Sandilands A, Chao SC, Lee JY, Sheu HM, McLean WH, Shimizu H. Analysis of Taiwanese ichthyosis vulgaris families further demonstrates differences in FLG mutations between European and Asian populations. Br J Dermatol. 2009; 161:448–451.
16. Kang TW, Lee JS, Oh SW, Kim SC. Filaggrin mutation c.3321delA in a Korean patient with ichthyosis vulgaris and atopic dermatitis. Dermatology. 2009; 218:186–187.
17. Zhang H, Guo Y, Wang W, Shi M, Chen X, Yao Z. Mutations in the filaggrin gene in Han Chinese patients with atopic dermatitis. Allergy. 2011; 66:420–427.
18. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
19. Lee SG, Kim BS, Kim JH, Lee SY, Choi SO, Shim JY, Hong TJ, Hong SJ. Gene-gene interaction between interleukin-4 and interleukin-4 receptor alpha in Korean children with asthma. Clin Exp Allergy. 2004; 34:1202–1208.
20. Nomura T, Akiyama M, Sandilands A, Nemoto-Hasebe I, Sakai K, Nagasaki A, Ota M, Hata H, Evans AT, Palmer CN, Shimizu H, McLean WH. Specific filaggrin mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J Invest Dermatol. 2008; 128:1436–1441.
21. Chen H, Common JE, Haines RL, Balakrishnan A, Brown SJ, Goh CS, Cordell HJ, Sandilands A, Campbell LE, Kroboth K, Irvine AD, Goh DL, Tang MB, van Bever HP, Giam YC, McLean WH, Lane EB. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol. 2011; 165:106–114.
22. Li M, Liu Q, Liu J, Cheng R, Zhang H, Xue H, Bao Y, Yao Z. Mutations analysis in filaggrin gene in northern China patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013; 27:169–174.
23. Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. FLG mutation p.Lys4021X in the C-terminal imperfect filaggrin repeat in Japanese patients with atopic eczema. Br J Dermatol. 2009; 161:1387–1390.
24. Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009; 129:682–689.
25. Ponińska J, Samoliński B, Tomaszewska A, Raciborski F, Samel-Kowalik P, Walkiewicz A, Lipiec A, Piekarska B, Komorowski J, Krzych-Fałta E, Namysłowski A, Borowicz J, Kostrzewa G, Majewski S, Płoski R. Filaggrin gene defects are independent risk factors for atopic asthma in a Polish population: a study in ECAP cohort. PLoS One. 2011; 6:e16933.
26. Bønnelykke K, Pipper CB, Tavendale R, Palmer CN, Bisgaard H. Filaggrin gene variants and atopic diseases in early childhood assessed longitudinally from birth. Pediatr Allergy Immunol. 2010; 21:954–961.
27. Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001; 137:1079–1081.
28. Kezic S, O'Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, Caspers P, Kemperman PM, Puppels GJ, Sandilands A, Chen H, Campbell LE, Kroboth K, Watson R, Fallon PG, McLean WH, Irvine AD. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012; 129:1031–1039.e1.
29. Kool M, Hammad H, Lambrecht BN. Cellular networks controlling Th2 polarization in allergy and immunity. F1000 Biol Rep. 2012; 4:6.
30. Lesiak A, Kuna P, Zakrzewski M, van Geel M, Bladergroen RS, Przybylowska K, Stelmach I, Majak P, Hawro T, Sysa-Jedrzejowska A, Narbutt J. Combined occurrence of filaggrin mutations and IL-10 or IL-13 polymorphisms predisposes to atopic dermatitis. Exp Dermatol. 2011; 20:491–495.
31. Kezic S, O'Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, Kroboth K, Watson R, Rowland M, McLean WH, Irvine AD. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011; 66:934–940.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download