Abstract
Eosinophilic otitis media (EOM) shows a very high rate of association with asthma, and intractable otitis media involves marked eosinophil infiltration into the middle ear. The middle ear space is connected to the nasopharynx by the Eustachian tube, and it is considered a part of the upper respiratory tract. Allergic rhinitis and asthma often coexist as chronic inflammatory diseases of the upper and lower airways, respectively, and have an impact on each other. In fact, inhaled corticosteroids reduce seasonal eosinophilia systemically in the circulation and locally in the nasal mucosa, as well as attenuate seasonal nasal symptoms. We report a case of EOM associated with adult-onset asthma that improved following optimal asthma therapy after changing the treatment from inhaled fluticasone propionate (FP) (200 µg b.i.d.) to a combination of FP/salmeterol (250/50 µg b.i.d.). This result supports the hypothesis that EOM and asthma are closely linked, presenting as different manifestations of a similar disease syndrome.
Eosinophilic otitis media (EOM) is characterized by the accumulation of eosinophils in the middle ear effusion and middle ear mucosa and has a strong association with asthma.1 The middle ear is an extension of the upper airway tract that is-like the airways-lined with pseudostratified ciliated columnar epithelium and is aerated via a narrow orifice. Hence, it is tempting to speculate that the relationship between EOM and asthma might be compared with that of allergic rhinitis and asthma. In the concept of 'one airway, one disease,' in allergic rhinitis and asthma,2 most data indicate a systemic link between the upper and lower airways, involving the bloodstream and bone marrow.3,4 We hypothesized that optimizing asthma treatment in patients with EOM with comorbid asthma might be beneficial for the treatment of EOM.
A 58-year-old Japanese female patient with adult-onset asthma first visited our ear, nose, and throat clinic at Tokyo Women's Medical University in August 1999 at age 46 years with complaints of a feeling of fullness in both ears that started in July 1999. On examination, we observed glue like otorrhea from the edges of the bilateral perforated tympanic membranes (Fig. 1). Marked eosinophilia was detected in the sticky otorrhea and the tympanic cavity granulomas. The peripheral blood eosinophil percentage was 8.0%, and the serum IgE level was 56 IU/mL. Capsulated hydrophobic carrier polymer-radioallergosorbent tests were positive only for Japanese cedar pollen. We diagnosed the patient with EOM. Treatment was started and continued for 11 years by instilling 0.1% betamethasone disodium phosphate solution (Rinderon) into the bilateral tympanic cavities. However, her ear symptoms did not improve after this treatment. She was diagnosed with asthma at age 43 years (1996) shortly after the first asthma attack. The diagnosis of asthma was made by a respiratory physician based on a comprehensive history of repeated bouts of coughing, wheezing, breathlessness, and chest tightness, as well as a clinical examination, including chest auscultation and pulmonary function tests. Her asthma was treated with montelukast (10 mg once daily) and inhaled fluticasone propionate (FP; 200 µg twice daily). Three years later, at the time of her first visit to the Department of Otolaryngology, she was still taking montelukast and inhaled FP therapy for her asthma, and we continued this regimen.
Despite the asthma treatment with inhaled FP (200 µg, b.i.d.), she continued to have two to three asthma attacks per year. She again consulted a respiratory specialist at the age of 57 years (July 2010), and the asthma treatment was stepped up to a combination of inhaled FP (250 µg) and salmeterol 50 µg (FSC) (Advair Diskus® 250/50; GlaxoSmithKline). Montelukast was continued at 10 mg/day, but the intratympanic instillation of 0.1% betamethasone disodium phosphate solution (Rinderon) was discontinued. Two months after changing the treatment to the combination of inhaled steroid and long-acting β2 agonists, her asthma was under control, and both the otorrhea and granulomas disappeared (Fig. 1). In September 2010, the percentage of peripheral blood eosinophils decreased to 3.2%, whereas the level of serum IgE remained largely unchanged at 55.1 IU/mL. Conductive hearing loss, which had been detected by pure-tone audiometry performed prior to the introduction of this combination therapy, improved thereafter (Fig. 2). The soft-tissue shadows assessed by computed tomography also improved following the stepped-up asthma treatment (Fig. 3). For more than 1 year thereafter, there has been no recurrence of otorrhea or granuloma formation and no deterioration of the acuity of hearing.
Tomioka et al.5 in 1993 reported that the otorrhea of patients with refractory otitis media accompanied by asthma was sticky and contained numerous eosinophils. In 2011, Iino et al.1 published diagnostic criteria for EOM. They proposed that patients with EOM or chronic otitis media with eosinophil-dominant effusion who also manifested two or more among the highest four items can be diagnosed as having EOM.
It was reported that approximately 90% of patients with EOM also have asthma.1 Like asthma, EOM is associated with local production of helper T type 2 (Th2) cytokines, such as interleukin (IL)-5.6 Thus, the relationship between EOM and asthma falls within the category of airway inflammatory diseases that are similar to the 'one airway, one disease' concept.
In the concept of 'one airway, one disease' applied to allergic rhinitis and asthma, most findings indicate a systemic link between the upper and lower airways involving the bloodstream and bone marrow.3,4 It was demonstrated that allergen inhalation by sensitized mice leads to a systemic increase in the levels of IL-5, a well-known growth and differentiation factor for eosinophils at sites of inflammation.7 Wang et al.8 reported that circulating IL-5 plays a crucial role in the migration of eosinophils toward the airways. Additionally, IL-5 is involved in eosinopoiesis in the bone marrow. Furthermore, segmental bronchial provocation in non-asthmatic allergic rhinitis patients increased peripheral blood IL-5 as well as nasal IL-5 levels.9,10 Conversely, it was reported that nasal allergen challenge in allergic rhinitis patients increased sputum eosinophil numbers, possibly by the action of peripheral blood IL-5.11 Interestingly, in allergic rhinitis patients, orally inhaled steroid reduced eosinophilia in both the peripheral blood and nose along with attenuation of nasal symptoms.12
Topical glucocorticosteroid treatment, which is increasingly becoming a first-line option for asthma and rhinitis, is thought to reflect topical airway drug action.13 Particular care was taken to avoid middle ear deposition of inhaled FSC in the present study. Patients were instructed to breathe through the mouth for at least 10 seconds after each administration. Accordingly, we believe it is unlikely that the present effects of FSC were due to direct middle ear deposition of FSC.
FP is a potent topically active corticosteroid that has been licensed for use by inhalation. Although it has previously been shown that FP causes suppression of adrenocortical activity even at clinically recommended doses, the selected dose of inhaled FP (250 µg b.i.d.), which is contained in FSC, or even FSC (250/50 µg b.i.d.), is considered not to provoke any clinically important adverse reactions, such as hypothalamic-pituitary-adrenal-axis impairment, in adults.14 Preclinical work has shown that FP and salmeterol have complementary mechanisms of action, and they also interact synergistically.15 One may speculate that such actions make FP more effective in reducing lower airway Th2 type inflammation, resulting in reduced communication between the bronchial airways and bone marrow (involving cells such as Th2 cells and cytokines such as IL-5), potentially promoting 'pan-airway' eosinophilia that could in this case have been abrogated by the inhaled FSC.
Recently, systemic and topical corticosteroids have been reported to be effective for EOM.16 Other treatments, such as a combination of anti-histamines and leukotriene receptor antagonists in addition to topical corticosteroids, also result in the relief of subjective symptoms. Patients with EOM often have co-morbid asthma. Although this is a single case history, we hypothesize that the improvement in EOM was due to better asthma control via step up of the inhaled therapy for asthma. Further studies with greater numbers of patients are needed. We suggest that optimized asthma therapy leading to better asthma control may be beneficial for the treatment of EOM in patients with co-morbid asthma.
Figures and Tables
Fig. 1
Tympanic membrane findings (right ear). (A) Prior to optimizing asthma treatment (i.e., prior to using the combination of inhaled FSC). Glue-like otorrhea is observed exuding from the tympanic membrane perforation. (B) Following optimized asthma treatment (i.e., after starting FSC). The glue-like otorrhea exuding from the tympanic membrane perforation has disappeared.
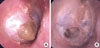
Fig. 2
Audiometry. Yellow color: prior to optimizing asthma treatment. Blue color: following optimized asthma treatment.

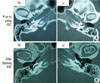
Fig. 3
Computed tomography images. (A) Prior to optimizing asthma treatment (i.e., prior to using FSC). Soft-tissue shadows are observed extending from the Eustachian tube to the tympanic cavity. (B) Following optimized asthma treatment (i.e., after starting FSC). The soft-tissue shadow in the Eustachian tube is reduced, whereas the shadow in the tympanic cavity has disappeared. FSC, fluticasone propionate 250 µg and salmeterol 50 µg.
References
1. Iino Y, Tomioka-Matsutani S, Matsubara A, Nakagawa T, Nonaka M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx. 2011. 38:456–461.
2. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. World Health Organization. GA(2)LEN. AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008. 63:Suppl 86. 8–160.
3. Pawankar R. Allergic rhinitis and asthma: are they manifestations of one syndrome? Clin Exp Allergy. 2006. 36:1–4.
4. Braunstahl GJ, Hellings PW. Allergic rhinitis and asthma: the link further unraveled. Curr Opin Pulm Med. 2003. 9:46–51.
5. Tomioka S, Yuasa R, Iino Y. Mogi G, Honjo I, Ishii T, Takasaka T, editors. Intractable otitis media in cases with bronchial asthma. Recent advances in otitis media. Proceedings of the second extraordinary international symposium on recent advances in otitis media. 1993. Amsterdam, New York: Kugler Publications;183–186.
6. Iino Y, Kakizaki K, Katano H, Saigusa H, Kanegasaki S. Eosinophil chemoattractants in the middle ear of patients with eosinophilic otitis media. Clin Exp Allergy. 2005. 35:1370–1376.
7. Hellings PW, Hessel EM, Van Den Oord JJ, Kasran A, Van Hecke P, Ceuppens JL. Eosinophilic rhinitis accompanies the development of lower airway inflammation and hyper-reactivity in sensitized mice exposed to aerosolized allergen. Clin Exp Allergy. 2001. 31:782–790.
8. Wang J, Palmer K, Lŏtvall J, Milan S, Lei XF, Matthaei KI, Gauldie J, Inman MD, Jordana M, Xing Z. Circulating, but not local lung, IL-5 is required for the development of antigen-induced airways eosinophilia. J Clin Invest. 1998. 102:1132–1141.
9. Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000. 161:2051–2057.
10. Braunstahl GJ, Overbeek SE, Fokkens WJ, Kleinjan A, McEuen AR, Walls AF, Hoogsteden HC, Prins JB. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001. 164:858–865.
11. Beeh KM, Beier J, Kornmann O, Meier C, Taeumer T, Buhl R. A single nasal allergen challenge increases induced sputum inflammatory markers in non-asthmatic subjects with seasonal allergic rhinitis: correlation with plasma interleukin-5. Clin Exp Allergy. 2003. 33:475–482.
12. Greiff L, Andersson M, Svensson C, Linden M, Wollmer P, Brattsand R, Persson CG. Effects of orally inhaled budesonide in seasonal allergic rhinitis. Eur Respir J. 1998. 11:1268–1273.
13. Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993. 148:S1–S26.
14. Chapman KR, Ringdal N, Backer V, Palmqvist M, Saarelainen S, Briggs M. Salmeterol and fluticasone propionate (50/250 microg) administered via combination Diskus inhaler: as effective as when given via separate Diskus inhalers. Can Respir J. 1999. 6:45–51.
15. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002. 19:182–191.
16. Iino Y, Nagamine H, Kakizaki K, Komiya T, Katano H, Saruya S, Kodera K. Effectiveness of instillation of triamcinolone acetonide into the middle ear for eosinophilic otitis media associated with bronchial asthma. Ann Allergy Asthma Immunol. 2006. 97:761–766.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download