Abstract
Purpose
We examined whether fractional exhaled nitric oxide (FeNO) levels are associated with atopy profiles in terms of mono-sensitization and poly-sensitization in asthmatic children.
Methods
A total of 119 children underwent an assessment that included FeNO measurements, spirometry, methacholine challenge, and measurement of blood eosinophil count, serum total IgE, and serum eosinophil cationic protein (ECP). We also examined sensitization to five classes of aeroallergens (house dust mites, animal danders, pollens, molds, and cockroach) using skin prick testing. The children were divided into three groups according to their sensitization profiles to these aeroallergens (non-sensitized, mono-sensitized, and poly-sensitized).
Results
The geometric means (range of 1 SD) of FeNO were significantly different between the three groups (non-sensitized, 18.6 ppb [10.0-34.7 ppb]; mono-sensitized, 28.8 ppb [16.6-50.1 ppb]; and poly-sensitized, 44.7 ppb [24.5-81.3 ppb], P=0.001). FeNO levels were correlated with serum total IgE concentrations, peripheral blood eosinophilia, and serum ECP levels to different degrees.
Conclusions
FeNO levels vary according to the profile of atopy, as determined by positive skin prick test results to various classes of aeroallergens. FeNO is also moderately correlated with serum total IgE, blood eosinophilia, and serum ECP. These results suggest that poly-sensitized asthmatic children may have the highest risk of airway inflammation.
Atopy and airway inflammation are fundamental features of asthma, however, the relationship between them is complex and reports on their association are conflicting.1 Atopy is defined as at least one positive reaction to allergens. The atopic population consists of two groups of individuals, those sensitized to only one class of aeroallergen (mono-sensitized) and those sensitized to multiple classes of allergen (poly-sensitized).2
After it became clear that chronic airway inflammation characterizes asthma, interest in noninvasive methods for measuring airway inflammation rapidly increased.3 Nitric oxide (NO) has been proposed as a marker of airway inflammation in asthma based on findings that fractional exhaled NO (FeNO) levels are higher in asthmatic children, which correlates with levels of eosinophilic inflammation in the airway mucosa.4,5 FeNO appears to be a useful clinical tool for assessing airway inflammation in asthma, with the advantages of being noninvasive and providing immediate results.6,7
While higher FeNO levels are known to occur in asthmatic children, some investigators have shown that this increase is also found in individuals with atopy.8 FeNO levels increase in atopic children regardless of whether they have asthma and are significantly higher than in non-atopic asthmatics.8 Although elevated FeNO levels have been observed in asthmatic children, this increase does not appear to be directly associated with asthma itself.
The fact that FeNO levels can be modified by other factors may lead to errors and raise concerns regarding the interpretation of increased FeNO levels in asthmatic children.9 Several studies have indicated that both the presence and degree of atopy are important factors for increased FeNO levels in asthmatic patients.10 Because of the high prevalence of atopy among asthmatic patients, it is important to determine the influence of these atopic conditions on FeNO. Although the atopic status itself has been found to be closely correlated with elevated FeNO levels, it is important to assess how strongly atopy affects FeNO levels in asthmatic children. The relationship between elevated FeNO levels and the degree of atopic sensitization requires clarification.
To date, the relationship between the presence of atopy and its profile in terms of mono-sensitization and poly-sensitization with FeNO, a validated indirect marker of airway inflammation, has not been clearly established. We evaluated the possible relationships between the presence and profiles of aeroallergen sensitization and FeNO levels in asthmatic children.
A total of 119 children with mild to moderate asthma, aged 10-13 years, were enrolled. All subjects had a history of episodic wheezing and/or dyspnea during the previous year, which was resolved with bronchodilators. The clinical severity of the asthma was assessed according to the National Education and Prevention Program criteria.11 Subjects were treated with inhaled short-acting β2-agonists on demand to relieve symptoms, with or without controller medications (inhaled corticosteroids, leukotriene receptor antagonists, or inhaled long-acting β2-agonists). All participants underwent a battery of tests, including FeNO, baseline spirometry, methacholine challenge tests, skin prick tests, and blood sampling at the Allergy Clinic and Environmental Health Center for Childhood Asthma of Korea University Anam Hospital. Subjects with concomitant allergic rhinitis after careful review of medical records were excluded. Also excluded were subjects with any history of symptoms suggestive of allergic rhinitis, such as recurrent symptoms of sneezing, rhinorrhea, and nasal stuffiness or itching, with the exception of the common cold, during the year prior to the study or when allergic rhinitis had been diagnosed by a physician. Patients with a history of near-fatal asthma, major exacerbations necessitating the use of systemic corticosteroids, or with serious respiratory diseases other than asthma were also excluded.
Parents gave written informed consent for their children to participate in the study. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (No. ED12055).
Spirometry (forced expiratory volume in 1 second [FEV1] and forced vital capacity [FVC]) was performed using a computerized spirometer (Microspiro-HI 298, Chest; Tokyo, Japan) in accordance with the recommendations of the American Thoracic Society.12 All subjects were asked to perform spirometry in the standard manner, and were required to have an FEV1 of at least 70% of the predicted value.
After obtaining baseline spirometry values, all subjects underwent bronchoprovocation with increasing concentrations of methacholine. The methacholine inhalation test was performed using a modified method of that described by Chai et al.13 Patients had been free of acute respiratory tract infections and asthma exacerbations for a minimum of 4 weeks prior to the test. All patients were asked to discontinue using inhaled short-acting β-agonists for 24 hours and inhaled long-acting β-agonists, leukotriene modifier, and corticosteroids for 7 days prior to testing. Methacholine solutions (Sigma Diagnostics, St. Louis, MO, USA) were prepared at different concentrations (0.075, 0.15, 0.3, 0.625, 1.25, 2.5, 5, 10, and 25 mg/mL) in a buffered saline solution (pH 7.4). A Rosenthal-French dosimeter (Laboratory for Applied Immunology, Baltimore, MD, USA), triggered by a solenoid valve set to remain open for 0.6 sec, was used to generate aerosols from a DeVilbiss 646 nebulizer (DeVilbiss Health Care, Somerset, PA, USA) with pressurized air at 20 psi. Each patient inhaled five inspiratory capacity breaths of the buffered saline solution and increasing concentrations of methacholine at 5-min intervals. FEV1 and FVC were measured 90 seconds after inhalation at each concentration, and the largest three FEV1 or FVC measurements were analyzed. The procedure was terminated when FEV1 decreased by more than 20% of its post-saline value or when the highest methacholine concentration (25 mg/mL) was reached. Percentage declines in FEV1 from the post-saline value were plotted against log concentrations of inhaled methacholine. The provocative concentration of methacholine (PC20) producing a 20% fall in FEV1 was calculated by interpolating between two adjacent data points.
FeNO was measured using a chemiluminescence analyzer (NIOX analyzer, Aerocrine, Sweden) during single-breath exhalation according to the ERS/ATS recommendations.14 We followed the manufacturer's recommendations: inhalation of NO-free air to total lung capacity, immediately followed by full exhalation against a positive mouthpiece counter pressure at a flow rate of 50 mL/sec into an on-line chemiluminescence analyzer to avoid any nasal contamination. Three measurements were made, and were considered validated when <10% variability was obtained. Each subject had been free of acute respiratory tract infections for at least 4 weeks prior to the measurement.
Skin prick testing was performed using five classes of 13 common aeroallergens: house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), animal danders (cat epithelium and dog epithelium), pollens (mugwort, ryegrass, ragweed, hazel, alder, and oak), molds (Aspergillus fumigates and Alternaria alternata), and cockroach (Blatella germanica). The allergens were supplied by Allergopharma (Reinbek, Germany). A mean wheal diameter >3 mm in the absence of any reaction to the negative control was considered to indicate a positive reaction.15 Atopy was defined as the presence of at least one positive reaction to these allergens. As for mono-sensitization and poly-sensitization, atopic subjects are frequently sensitized to more than one allergen belonging to clusters of allergen classes.16,17 Thus, subjects sensitized to only one class of allergens were considered to be mono-sensitized, while those sensitized to two or more classes of allergens were considered to be poly-sensitized.
Serum total IgE levels were measured using a Coat-A-Count Total IgE IRMA (Diagnostic Products Co., Los Angeles, CA, USA) according to the manufacturer's instructions. The number of peripheral blood eosinophils was counted in blood samples containing EDTA using an automated hematology analyzer (Coulter Counter STKS, Beckman Coulter, Fullerton, CA, USA). Serum eosinophil cationic protein (ECP) levels were measured using a commercially available fluoroimmunoassay kit (Pharmacia ECP UniCAP System FEIA, Pharmacia Diagnostics, Uppsala, Sweden) with a detection limit <2.0 µg/L.
FEV1 and FVC are expressed as percent predicted values based on the data from our local population. The values for FeNO, methacholine PC20, serum total IgE levels, blood eosinophil counts, and serum ECP levels were log transformed before statistical analysis. Data are presented as mean±SD or geometric mean (range of 1 SD), as appropriate. The variables were compared between the three groups using one-way analysis of variance (ANOVA) and the post hoc Tukey's honestly significant difference test or the chi-square test for multiple comparisons, as appropriate. Correlations between FeNO and serum total IgE levels, blood eosinophil counts, serum ECP levels, or various pulmonary function parameters were calculated using a linear regression model. All statistical analyses were performed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered to be statistically significant.
The clinical characteristics of the three groups are shown in Table 1. Of the 119 children, 32 were designated as non-sensitized, 46 were mono-sensitized, and 41 were poly-sensitized. The mean ages, BMIs, and the gender distribution were not significantly different between the three groups. The geometric means (range of 1 SD) of serum total IgE were significantly different between the three groups: 380.2 IU/mL (128.8-1122.0 IU/mL) in poly-sensitized subjects, 302.0 IU/mL (107.2-851.1 IU/mL) in mono-sensitized subjects, and 107.2 IU/mL (32.4-354.8 IU/mL) in non-sensitized subjects (P=0.019). The blood eosinophil counts and serum ECP concentrations were also different between the three groups, with marginal significance (P=0.011 and P=0.093, respectively).
Table 2 shows the pulmonary function parameters of the subjects. The baseline FEV1, FVC, and FEV1/FVC levels were not significantly different between the three groups (P=0.781, P=0.862, and P=0.260, respectively). However, the geometric mean (range of 1SD) of methacholine PC20 was significantly lower in poly-sensitized subjects (2.51 mg/mL [0.34-18.6 mg/mL]) than in the other two groups (mono-sensitized subjects, 6.46 mg/mL [1.26-33.1 mg/mL]; non-sensitized subjects, 14.1 mg/mL [2.14-93.3 mg/mL]) (P=0.001).
We found increased FeNO levels in the atopic asthmatic children (36.3 ppb [range of 1 SD, 20.4-64.6 ppb]) compared to non-atopic asthmatics (18.6 ppb [range of 1 SD, 10.0-34.7 ppb]) (P=0.001). There were also significant differences in FeNO values between the two sensitized groups (mono-sensitized, 28.8 ppb [range of 1 SD, 16.6-50.1 ppb] versus poly-sensitized, 44.7 ppb [range of 1 SD, 24.5-81.3 ppb]) (P=0.026; Figure).
We also analyzed possible correlations between FeNO values and various biological markers of asthma. FeNO levels correlated significantly with serum total IgE levels and blood eosinophils, and displayed inverse relationships with FEV1, FVC, FEV1/FVC, and methacholine PC20 (Table 3).
We found significant relationships between FeNO levels and the degree of sensitization to aeroallergens in asthmatic children. We noted a weak but significantly greater elevation of FeNO levels in poly-sensitized asthmatics than in mono-sensitized asthmatics. FeNO levels were correlated with serum total IgE levels, blood eosinophils, serum ECP levels, and some spirometric parameters, to varying extents. These results are consistent with those of previous studies, and suggest that FeNO may be moderately correlated with the profiles of atopic sensitization and allergic inflammation.
Although several studies have shown that asthma and/or atopy are closely related to FeNO,9,10 these relationships are inconsistent in clinically selected samples.18,19 A previous study of 222 asthmatic children showed that FeNO levels are elevated in some, but not all children with atopic asthma.7 Prasad et al.8 demonstrated that FeNO levels are higher in atopic children than in non-atopic asthmatic children and non-atopic, non-asthmatic children in a study with a large pediatric asthmatic population. Furthermore, they showed that non-atopic children have no significant difference in FeNO levels whether they are asthmatic or not.
Previous studies suggested that the degree of atopy is associated with FeNO levels, and that increasing FeNO levels are related to the number of positive skin prick test results in asthmatic children.20 However, Moore et al.21 demonstrated that FeNO is not always associated with the number of positive skin test responses, blood eosinophils, or serum IgE levels in asthmatics. FeNO displays even greater independence from asthma and asthma-like symptoms after controlling for atopy. The relationship between FeNO and asthma is complex, and the mechanisms for increased FeNO levels require further investigation.
We divided the asthmatic children into three groups according to their different profiles of atopic sensitization. It was hypothesized that atopic sensitization could be a marker of airway inflammation in asthmatic children. This hypothesis is supported by a previous study, which reported correlations between serum total IgE levels and FeNO concentrations in asthmatics.7 However, it is problematic to explain airway inflammation in asthmatics by the presence of atopy alone. The degree of atopic sensitization is more important than simply the presence of atopy in further elucidation of the relationship between FeNO levels and atopy.
We also found a positive relationship between FeNO levels and atopy profiles, which is in agreement with previous studies. The relationship between FeNO levels and the degree of atopic sensitization potentially indicates that allergen sensitization may contribute to atopic airway inflammation in asthmatic subjects. A significant correlation between atopy scores and the severity of exercise-induced bronchoconstriction, which has been shown to be correlated with the markers of eosinophilic airway inflammation,22 also supports our contention that the degree of allergen sensitization may contribute to allergic airway inflammation. In contrast, Silvestri et al.23 did not find significant differences in the FeNO levels between mono-sensitized and poly-sensitized asthmatic children. This discrepancy may be explained by the small number of mono-sensitized children in that study.
Our results indicate that aeroallergen sensitization clearly plays an important role in determining FeNO levels in asthmatic children. Thus, it could be speculated that airway eosinophilic inflammation may increase with the degree of atopy. Although the mechanism by which an increase in the degree of atopic responsiveness induces a rise in FeNO is not fully understood, it is becoming evident that NO production correlates more specifically with airway inflammation where eosinophilia and clinical atopic features dominate.8 An increasing degree of atopic responsiveness leads to greater cellular activation with inflammation, and consequently upregulated production of inducible nitric oxide synthase (iNOS).4 Elevated FeNO levels are thought to result from increased expression and activity of iNOS in airway epithelial and inflammatory cells.24,25 In atopic asthmatics, the degree of atopic sensitization appears to reflect systemic (blood eosinophilis) and organ-specific (FeNO) markers of allergic inflammation.7,26
In the present study, the elevated FeNO concentrations in the poly-sensitized subjects reflected the presence of clinical inflammation and induction of iNOS by inflammatory cytokines.27 Inhaled allergens are thought to be an important cause of ongoing inflammation in the lungs of sensitized children who develop asthma.28 In the present study, exposure to inhaled allergens in the sensitized groups may have contributed to the elevated FeNO.
FeNO is significantly correlated with atopy in children with recent exposure to allergens, independent of symptoms, and during remission, asymptomatic adolescents with atopic asthma show elevated FeNO levels with increased eosinophilic activity in the biopsies of bronchial mucosa.29 Airway eosinophilia is a common feature of atopy, and FeNO levels may determine the clinical expression of atopy because atopy can be considered an immune disorder associated with increased airway inflammation.30
In contrast, we found an increasing tendency towards higher ECP in poly-sensitized than in mono-sensitized subjects, without statistical significance. Our borderline association between serum ECP concentrations and FeNO levels suggests that both markers participate in eosinophilic inflammation in asthmatics. ECP is a potent biomarker of asthma and has been used to assess inflammation in asthmatic children. FeNO has been reported to be well correlated with ECP concentrations in sputum4 or in bronchoalveolar lavage fluid.5 Thomas et al.4 reported that FeNO levels were significantly correlated with sputum ECP concentrations in a cohort of Australian children and had a positive relationship with sputum eosinophilia. In contrast, Piacentini et al.18 found no significant correlation between FeNO levels and serum ECP concentrations. Furthermore, although serum ECP levels are higher in children with atopic asthma, the wide ranges are likely to limit the relevance of measuring serum ECP levels in children as a guide to the diagnosis or management of asthma.31 The measurement of FeNO is easier and more reliable than that of serum ECP in pediatric patients, because the effort and coordination required are minimal and more organ-specific. Indeed, FeNO is a more sensitive indicator of disease severity than circulating markers of inflammation, such as serum ECP.27
In the present study, the non-sensitized subjects showed relatively high serum total IgE levels. It should be acknowledged that atopy is not always detectable by skin tests. We did not obtain sensitization profiles for food allergens or use other definitions of atopy, such as specific IgE levels or atopic scores. Therefore, it is possible that some of our non-sensitized subjects may have belonged in the sensitized group. In addition to subjects with the highest poly-sensitized levels, we enrolled mild to moderate asthmatic subjects. However, asthma severity itself might be associated with the degree of sensitization or FeNO levels. Investigators should be aware that asthma severity is one of the important confounding factors. Moreover, although our subjects were asked to cease medication 1 week prior to the test, FeNO could still be affected by asthma medications.
Some investigators have indicated elevated FeNO levels in subjects with allergic rhinitis other than asthmatics.19,32 In the present study, this is unlikely to have influenced the results of our analysis, because we excluded asthmatic patients who had concomitant allergic rhinitis symptoms.
In summary, FeNO levels were associated with both the presence and profiles of atopic sensitization as determined by positive skin prick test results to various classes of aeroallergens in asthmatic children. Meaningful interpretation of FeNO may only be possible when the presence and the degree of sensitization are considered.33 FeNO is a good biomarker for the severity of atopic inflammation, which indicates that poly-sensitized and/or strongly sensitized children are at a high risk of airway inflammation.
Figures and Tables
Figure
Mean and percentile (10th, 25th, 75th, and 90th) distributions of FeNO in non-sensitized, mono-sensitized, and poly-sensitized asthma groups.
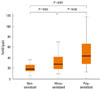
Table 1
Clinical characteristics of the asthmatic children

Table 2
Pulmonary function parameters in asthmatic children

Table 3
Linear regression coefficients for fractional exhaled nitric oxide in children with asthma
ACKNOWLEDGMENTS
This study was supported in part by grants from the Environmental Health Center for Childhood Asthma (2012), Ministry of Environment, Korea; the Korean Health Technology R&D Project, Ministry of Health and Welfare, Korea (A110663); and the Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology (MEST), Korea (2012R1A1A3014036).
References
1. Høst A, Halken S. The role of allergy in childhood asthma. Allergy. 2000. 55:600–608.
2. Silvestri M, Oddera S, Rossi GA, Crimi P. Sensitization to airborne allergens in children with respiratory symptoms. Ann Allergy Asthma Immunol. 1996. 76:239–244.
3. Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of Southern California schoolchildren. Respir Res. 2009. 10:28.
4. Thomas PS, Gibson PG, Wang H, Shah S, Henry RL. The relationship of exhaled nitric oxide to airway inflammation and responsiveness in children. J Asthma. 2005. 42:291–295.
5. Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, Shields MD. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002. 57:383–387.
6. Cordeiro D, Rudolphus A, Snoey E, Braunstahl GJ. Utility of nitric oxide for the diagnosis of asthma in an allergy clinic population. Allergy Asthma Proc. 2011. 32:119–126.
7. Banovcin P, Jesenak M, Michnova Z, Babusikova E, Nosal S, Mikler J, Fabry J, Barreto M. Factors attributable to the level of exhaled nitric oxide in asthmatic children. Eur J Med Res. 2009. 14:Suppl 4. 9–13.
8. Prasad A, Langford B, Stradling JR, Ho LP. Exhaled nitric oxide as a screening tool for asthma in school children. Respir Med. 2006. 100:167–173.
9. Hervás D, Milán JM, Garde J. Differences in exhaled nitric oxide in atopic children. Allergol Immunopathol (Madr). 2008. 36:331–335.
10. Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006. 118:1218–1225.
11. National Asthma Education and Prevention Program. National Institutes of Health publication 97-4051. Guidelines for the diagnosis and management of asthma: expert panel report 2. 1997. Bethesda, MD: US Department of Health and Human Services.
12. American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995. 152:1107–1136.
13. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.
14. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005. 171:912–930.
15. Høst A, Andrae S, Charkin S, Diaz-Vázquez C, Dreborg S, Eigenmann PA, Friedrichs F, Grinsted P, Lack G, Meylan G, Miglioranzi P, Muraro A, Nieto A, Niggemann B, Pascual C, Pouech MG, Rancé F, Rietschel E, Wickman M. Allergy testing in children: why, who, when and how? Allergy. 2003. 58:559–569.
16. Storm van's Gravesande K, Moseler M, Kuehr J. The most common phenotypes of sensitization to inhalant allergens in childhood. Clin Exp Allergy. 1997. 27:646–652.
17. Yoo Y, Yu J, Kim DK, Choi SH, Koh YY. Coincidence of atopy and its profile (monosensitization/polysensitization) between sibling pairs. Ann Allergy Asthma Immunol. 2005. 95:433–437.
18. Piacentini GL, Bodini A, Costella S, Suzuki Y, Zerman L, Peterson CG, Boner AL. Exhaled nitric oxide, serum ECP and airway responsiveness in mild asthmatic children. Eur Respir J. 2000. 15:839–843.
19. Malmberg LP, Petäys T, Haahtela T, Laatikainen T, Jousilahti P, Vartiainen E, Mäkelä MJ. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol. 2006. 41:635–642.
20. Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, Hodgdon K, Morgan W, Sorkness CA, Lemanske RF Jr. Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003. 112:883–892.
21. Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE. National Heart, Lung, Blood Institute's Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007. 119:405–413.
22. Koh YI, Choi IS, Lim H. Atopy may be related to exercise-induced bronchospasm in asthma. Clin Exp Allergy. 2002. 32:532–536.
23. Silvestri M, Sabatini F, Spallarossa D, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Exhaled nitric oxide levels in non-allergic and allergic mono- or polysensitised children with asthma. Thorax. 2001. 56:857–862.
24. Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001. 163:1693–1722.
25. Marshall HE, Stamler JS. NO waiting to exhale in asthma. Am J Respir Crit Care Med. 2000. 161:685–687.
26. Bommarito L, Migliore E, Bugiani M, Heffler E, Guida G, Bucca C, de Marco R, Rolla G. ECRHS Turin, Italy Study Group. Exhaled nitric oxide in a population sample of adults. Respiration. 2008. 75:386–392.
27. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999. 159:69–73.
28. Duff AL, Platts-Mills TA. Allergens and asthma. Pediatr Clin North Am. 1992. 39:1277–1291.
29. van den Toorn LM, Prins JB, de Jongste JC, Leman K, Mulder PG, Hoogsteden HC, Overbeek SE. Benefit from anti-inflammatory treatment during clinical remission of atopic asthma. Respir Med. 2005. 99:779–787.
30. Malmberg LP, Turpeinen H, Rytilä P, Sarna S, Haahtela T. Determinants of increased exhaled nitric oxide in patients with suspected asthma. Allergy. 2005. 60:464–468.
31. Joseph-Bowen J, de Klerk N, Holt PG, Sly PD. Relationship of asthma, atopy, and bronchial responsiveness to serum eosinophil cationic proteins in early childhood. J Allergy Clin Immunol. 2004. 114:1040–1045.
32. Chiron R, Vachier I, Khanbabaee G, Molinari N, Varrin M, Godard P, Chanez P. Impact of rhinitis on asthma control in children: association with FeNO. J Asthma. 2010. 47:604–608.
33. Franklin PJ, Stick SM, Le Souëf PN, Ayres JG, Turner SW. Measuring exhaled nitric oxide levels in adults: the importance of atopy and airway responsiveness. Chest. 2004. 126:1540–1545.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download