Abstract
Purpose
To evaluate the frequency of banana sensitization and allergy among a group of atopic Egyptian children in relation to parental/self reports.
Methods
This is a case-control study included 2 groups of allergic children with and without history of banana allergy, each included 40 patients. They were subjected to skin prick test (SPT) using commercial banana allergen extract and prick-prick test (PPT) using raw banana, in addition to measuring the serum banana-specific IgE. Oral banana challenge was performed in suspected cases.
Results
Banana allergy was diagnosed in 3 (7.5%) patients based on positive history of allergy on exposure to banana, positive SPT/PPT and elevated banana-specific IgE. The 3 patients had bronchial asthma with exacerbation upon banana exposure. The PPT results conform with those of SPT both in diagnosis of banana allergy and in the skin reactivity to banana. Serum banana-specific IgE was detectable in the whole studied sample with higher serum level among those without history of banana allergy (P=0.005). Oral banana challenge was negative for 20 patients with history of banana allergy and positive serum banana-specific IgE but negative SPT and PPT.
Conclusions
Self/parental reports of banana allergy is high while the actual banana allergy is uncommon. The PPT seems as reliable as SPT in diagnosis of banana allergy unlike specific IgE which reflects sensitization rather than allergy. Oral food challenge remains the most helpful tool for diagnosis of food allergy in suspected cases.
IgE antibody-mediated reactions to banana are typically mild, confined to the oral cavity, and related to initial sensitization to pollens that share homologous proteins with banana; this is known as the pollen-food syndrome or oral allergy syndrome.1 Banana also contains allergens in common with those in latex. Therefore, banana allergy often occurs in patients sensitized to latex or pollens and it is much less common in patients without latex allergy or pollinosis. However, these conclusions are based mostly on observations on banana allergy in adults, because banana allergy in infants appears to be rare.2
However, systemic reactions to banana may also occur. Severe reactions to banana have been associated with the presence of IgE antibody to particular proteins, such as lipid transfer proteins or storage proteins, that may be more stable and/or to which sensitization may have occurred through the gastrointestinal route in contrast to the milder symptoms attributed to reactions to less stable allergens such as profilin.3
An early frequent exposure to banana allergens was considered a possibility factor for the development of banana sensitization.4 Patient may have been sensitized to banana by ingestion of breast milk, therefore, the allergenic components causing banana allergy and the route of sensitization to banana in infants may differ from those in adults; it seems that oral sensitization to food occurs more easily in infants than adults because gut mucosal defence including immune system is undeveloped in infants.5
The diagnosis of food allergy relies upon history, skin prick test (SPT), specific IgE estimation, and if necessary oral challenge test. Prick-prick testing (PPT) with fresh fruit is a quick and inexpensive method to increase the diagnostic yield of SPT. Any patient with a convincing history and negative commercial extract SPT should have PPT.6
We aimed to explore the frequency of banana allergy among a group of Egyptian children with allergic disorders as diagnosed by SPT, PPT and serum banana specific IgE in relation to positive history of banana allergy.
This case-control study comprised 80 children with physician-diagnosed allergic diseases, where 40 children with history of banana allergy were compared to an equal number of children without such a history. An informed verbal consent was obtained from the parents or care-givers before enrollment. The study protocol gained the acceptance of the local ethics committee of the Pediatric Department.
Detailed history was taken with special emphasis on the onset of banana exposure, and whether exposure to banana triggers the allergic exacerbation, history of latex allergy, other triggers and family history of allergy. General examination as well as chest, skin, and ENT examination were performed for each patient to verify the diagnosis.
Serum total IgE was measured by enzyme linked immunosorbent assay (ELISA) (Medix Biotech, Inc., Agenzyme Company, Industrial Road, San Carios, CA, USA). A serum IgE level was considered elevated if it exceeded the highest reference value for age.
Serum specific IgE assay for banana by the ELISA technique (RIDASCREEN specific IgE, R-Biopharm AG, Darmstadt, Germany). According to the manufacturer's recommendations, detectable banana specific IgE was considered at levels above 0.35 IU/mL. Increased levels when banana specific IgE was from 0.7 to 3.49 IU/mL and very increased levels when banana specific IgE was from 3.5 to 99.99 IU/mL. Complete blood counting was done using an automated cell counter (Coulter MicroDiff 18, Fullerton, CA, USA) and differential counting was done manually.
SPT with commercial banana extract (Allergy Laboratories, Oklahoma City, OK, USA). First generation antihistamines were avoided for 72 hours before SPT, while second generation antihistamines were avoided at least 5 days before the procedure. The volar aspect of the patient's forearm was cleaned with alcohol, the test sites on the volar aspect of the forearm were marked and labeled at least 3 cm apart. The marked site was dropped by the allergen and gently pricked by sterile skin test lancet. Positive and negative control solutions were similarly applied. The patient is asked to wait for twenty minutes before interpretation of the results.
PPT of the skin was performed similarly for each patient in the same setting using raw banana. It was done by pricking the raw banana by sterile lancet and using the same lancet to prick the patient's skin.
A response of at least 3-mm diameter (with equivalent erythema) more than diluent control done at the same time is required.7
Open oral banana challenge (using two bananas) was carried out in only 20 (out of 37) patients, who had history of exacerbation of their allergic diseases in relation to eating banana, increased serum banana specific IgE but had negative SPT/PPT, and accepted to continue the study under close medical supervision for four hours, while taking all the precautions needed to prevent/treat anaphylaxis.
Analysis of data was done using SPSS (statistical program for social science) version 15 Numeric data were expressed as mean±standard deviation and median (range) when applicable; the qualitative data were expressed as frequency and percentage. Chi-square or Fisher exact test were used to examine the relation between qualitative variables. Comparison between two continuous variables was done using independent t student test for normally distributed variables and its non-parametric analogue, Mann Whitney test was used for not normally distributed ones.
All tests were 2 tailed, P values less than 0.05 were considered significant, and less than 0.001 were considered as highly significant.
The sex distribution was comparable among allergic children with history of banana allergy and those without such a history (P=0.3). Patients with history of banana allergy were significantly younger than those without this history (P=0.009; Table 1).
Bronchial asthma and acute urticaria were the two allergic disorders observed in both groups, however, bronchial asthma was found in the majority of the studied patients in the two groups. All patients without history of banana allergy had bronchial asthma, of whom, 3/40 (7.5%) had associated acute urticaria while 32/40 (80%) of those with positive history of banana allergy had bronchial asthma and acute urticaria was also present in 12/40 (30%) of these patients where it is associated with bronchial asthma in 7 patients.
The doses of inhaled corticosteroids in the studied asthmatic patients did not vary significantly among the 2 groups (P=0.6).
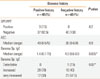
Positive SPT to banana was detected in only 3 (7.5%) allergic patients who had positive history of banana allergy. Of these patients, two were +3 and one patient was +2. The results of PPT agreed with the results and the degree of skin reactivity elicited by SPT in those patients. They were 2 males whose ages were 4 and 5 years and one female whose age was 4 years. All had controlled mild persistent bronchial asthma, with history of asthma exacerbation few minutes after banana ingestion.
All studied patients, whether SPT/PPT positive or negative had detectable specific IgE to banana (>0.35 IU/mL) according to the manufacturer's definition (Table 2). The allergic children without history of banana allergy had significantly higher banana specific IgE (P=0.005) and significantly higher frequency of very increased (3.50-99.99) levels (P=0.02) than those reporting a positive history. However, the frequency of increased banana-specific IgE (0.7 to 3.49 IU/mL) was higher (70%) among the group with positive history of banana allergy. The levels of banana specific IgE of the 2 patients with SPT/PPT of + 3 were 0.78 IU/mL and 4.5 IU/mL, while the level was 70 IU/mL in the patient with +2 SPT/PPT.
Open oral banana challenge (using two bananas) was intended to be performed to the 37 (92.5%) patients with positive history and positive serum specific IgE to banana but with negative banana SPT/PPT. However, only 20 patients consented to the procedure. The results were negative in all and there were no reported adverse effects to banana ingestion during the period of observation.
In the present study, 7.5% (n=3) of the allergic children had clinical banana allergy based on their positive history, positive SPT and elevated serum banana specific IgE. The prevalence of banana allergy from different parts of the world has been estimated to be 0.6%,8 0.4%,9 1.2%,10 0.04%,11 and 0.1%12 in the general population. The higher prevalence of banana allergy in our study is related to the studied population where only atopic children were enrolled. It has been reported that 67%13 and 40.6%14 of asthmatic patients had banana sensitization while banana allergy was detected in 5.6% of patients with atopic dermatitis.15
PPT was done to determine if the results of SPT are enhanced by the use of fresh food compared with the available commercial banana extract. We found that the results of PPT conformed 100% with the results and degree of skin reactivity elicited by SPT. This suggested that PPT could be used as an alternative to SPT in testing for banana allergy with apparently no increased risk of false results but this needs verification in larger scale studies and examining its applicability in different food allergies. In contrast, it has been reported that both the sensitivity and specificity of SPT are enhanced by the use of fresh food compared with the available commercial extracts.16 A PPT with fresh banana was found to be positive in a patient with positive history of atopic exacerbation on exposure to banana, although he had negative SPT.6 Moreover, the PPT results were significantly better matched with the results of DBPCFC than those using commercial allergen extracts. Also, the accuracy of SPT was markedly improved with fresh allergen extracts compared to that with commercial allergen extracts.17
However, a poor concordance between PPT and the oral food challenge test (OFC) was reported in 128 children with atopic dermatitis (AD), where 58 (45%) of their studied patients had positive PPT to banana, but only one child from 58 had positive OFC to banana (0.8%). This was attributed to the possible irritation of the skin of patients with AD by the raw fruit giving false positive results.18
Serum banana specific IgE was found to be elevated in the whole studied atopic children with and without banana allergy. Thus this represented a state of sensitization to banana rather than clinical banana allergy possibly related to the early introduction of banana in the food of infants. However, we could not trace this possibility through history taking except in one patient who was only 6 months old when bananas were introduced in his diet; otherwise the mothers of the studied children failed to recall the dietetic history of their children. Whether these patients with elevated specific IgE especially those with very increased level will develop banana allergy or remain in sensitization phase, this needs to be verified with long term follow up for these patients. It has been reported that, not every patient who has positive IgE test (and is therefore sensitized), has a clinically relevant allergy.19 A previous study demonstrated that 54 patients had negative oral challenges performed with cow's milk, hen eggs, wheat, peanuts, seafood, and/or fruit; although 46 of them had positive serum specific IgE.20 The increased frequency of increased and very increased serum banana specific IgE in both groups might reflect possible future banana allergy in these patients. Self-reported food allergy, although this does not represent actual food allergy, epidemiologically, it is useful as a proxy measure of the potential demand for allergy medical services, and may guide public health allergy service users between general and specialist medicine.21
Children with food allergy are two to four times as likely to experience other allergic conditions and asthma as children without food allergy.22 In the current study, the 3 patients with banana allergy had bronchial asthma with exacerbation of their asthma on exposure to banana. However, none of the studied patients had oral allergy syndrome (OAS), anaphylaxis, gastrointestinal symptoms or latex allergy. A previous study demonstrated that most of their studied children with food allergy (74.85%) had both rhinitis and asthma, 17.98% had asthma, 4.24% had rhinitis, and a small number of patients 1.32% had skin allergies, such as itching, eczema and urticaria.14
In our patients, allergy to banana as detected by SPT and PPT was not associated with more severe grades of bronchial asthma nor did it dictate an intake of higher doses of corticosteroids inhalation. This is in accordance with the results of a previous study which found that banana allergy did not affect the severity of bronchial asthma.13 The current study could not demonstrate a gender influence on the frequency of banana allergy. However, male predominance was observed in the whole studied sample, where most of our studied allergic patients were prepubertal. The younger age of the patients with history of banana allergy than those without such a history might reflect the parental concern about food allergy which could lead to overestimation of the condition as only 3 of these patients were found to have banana allergy.
Oral food challenges are occasions when results of primary in vivo and in vitro diagnostic tests (skin tests and IgE antibody serology) do not agree with each other or are not consistent with the history or other findings.23 In the present study, the negative open oral banana challenge in the 20 patients who reported allergic symptoms following ingestion of banana but had negative SPT indicated that false perception of food allergy is common among parents of atopic children. Moreover, it highlights the role of oral food challenge as a gold standard for the diagnosis of food allergy. It has been reported that, for fruit and vegetable allergy, prevalence based on perception (history) were generally higher than those based on sensitization.24 In a large cross-sectional survey where 20.4% of the respondents reported suffering from food intolerance, less than 2% actually experienced symptoms when subjected to a double blind, placebo-controlled food challenge.25 One study limitation is that oral challenge was not done to the whole studied sample, however in the 3 patients with positive SPT/PPT, we depended on the presence of all the following: positive history, positive SPT and specific IgE. While in suspected cases, oral challenge was performed in the subjects who consented to do it.
Thus, banana allergy appears to be uncommon among allergic Egyptian children; however, the sensitization rates were very high, raising the concern for possible future allergy. Self/parental reports of banana allergy were far higher than the actual status of banana allergy putting these children at risk for nutritional compromise because of food avoidance. Hence, a positive history should be confirmed by a positive SPT before prescribing elimination diets. Oral food challenge can help to rule out banana allergy in children with a positive history albeit a negative SPT. PPT proved as reliable as SPT for the diagnosis of banana allergy in this small scale study. However, its use in clinical practice in place of SPT awaits confirmation in larger scale studies.
Figures and Tables
Table 1
Demographic and clinical data of the studied patients
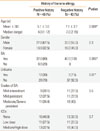
Table 2
Laboratory data of the studied patients
References
1. Ortolani C, Ispano M, Pastorello E, Bigi A, Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988. 61:47–52.
2. Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000. 106:27–36.
3. Beyer K, Grishina G, Bardina L, Grishin A, Sampson HA. Identification of an 11S globulin as a major hazelnut food allergen in hazelnut-induced systemic reactions. J Allergy Clin Immunol. 2002. 110:517–523.
4. Moreno-Ancillo A, Domínguez-Noche C, Gil-Adrados AC, Cosmes PM. Allergy to banana in a 5-month-old infant. Pediatr Allergy Immunol. 2004. 15:284–285.
5. Ito A, Ito K, Morishita M, Sakamoto T. A banana-allergic infant with IgE reactivity to avocado, but not to latex. Pediatr Int. 2006. 48:321–323.
6. Hauswirth DW, Burks AW. Banana anaphylaxis with a negative commercial skin test. J Allergy Clin Immunol. 2005. 115:632–633.
7. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, Sicherer S, Golden DB, Khan DA, Nicklas RA, Portnoy JM, Blessing-Moore J, Cox L, Lang DM, Oppenheimer J, Randolph CC, Schuller DE, Tilles SA, Wallace DV, Levetin E, Weber R. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008. 100:S1–S148.
8. van Bockel-Geelkerken M, Meulmeester JF. Prevalence of putative food hypersensitivity in young children. Ned Tijdschr Geneeskd. 1992. 136:1351–1356.
9. Brugman E, Meulmeester JF, Spee-van der Wekke A, Beuker RJ, Radder JJ, Verloove-Vanhorick SP. Prevalence of self-reported food hypersensitivity among school children in The Netherlands. Eur J Clin Nutr. 1998. 52:577–581.
10. Kristjansson I, Ardal B, Jonsson JS, Sigurdsson JA, Foldevi M, Björkstén B. Adverse reactions to food and food allergy in young children in Iceland and Sweden. Scand J Prim Health Care. 1999. 17:30–34.
11. Rancé F, Grandmottet X, Grandjean H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clin Exp Allergy. 2005. 35:167–172.
12. Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, Dean T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006. 117:1118–1124.
13. Aba-Alkhail BA, El-Gamal FM. Prevalence of food allergy in asthmatic patients. Saudi Med J. 2000. 21:81–87.
14. Mandal J, Das M, Roy I, Chatterjee S, Barui NC, Gupta-Bhattacharya S. Immediate hypersensitivity to common food allergens: an Investigation on food sensitization in respiratory allergic patients of Calcutta, India. World Allergy Organ J. 2009. 2:9–12.
15. Rokaite R, Labanauskas L, Vaideliene L. Role of the skin patch test in diagnosing food allergy in children with atopic dermatitis. Medicina (Kaunas). 2004. 40:1081–1087.
16. Rancé F, Juchet A, Brémont F, Dutau G. Correlations between skin prick tests using commercial extracts and fresh foods, specific IgE, and food challenges. Allergy. 1997. 52:1031–1035.
17. Kim TE, Park SW, Noh G, Lee S. Comparison of skin prick test results between crude allergen extracts from foods and commercial allergen extracts in atopic dermatitis by double-blind placebo-controlled food challenge for milk, egg, and soybean. Yonsei Med J. 2002. 43:613–620.
18. Cantani A, Micera M. The prick by prick test is safe and reliable in 58 children with atopic dermatitis and food allergy. Eur Rev Med Pharmacol Sci. 2006. 10:115–120.
19. Niggemann B, Beyer K. Diagnostic pitfalls in food allergy in children. Allergy. 2005. 60:104–107.
20. Ueno H, Yoshioka K, Matsumoto T. Usefulness of the skin index in predicting the outcome of oral challenges in children. J Investig Allergol Clin Immunol. 2007. 17:207–210.
21. Fiocchi A, Bouygue GR, Terracciano L, Sarratud T, Martelli A. Ruling out food allergy in pediatrics and preventing the "march" of the allergic child. Allergy Asthma Proc. 2006. 27:306–311.
22. Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007. 119:1016–1018.
23. Cohn JR, Bahna SL, Wallace DV, Goldstein S, Hamilton RG. AAAAI Work Group Report: Allergy Diagnosis in Clinical Practice [Internet]. updated 2006 Nov; cited 2011 Dec. Available from: http://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Allergy-Diagnosis-2006.pdf.
24. Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, Sodergren E, Dahlstrom J, Lindner T, Sigurdardottir ST, McBride D, Keil T. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008. 121:1210–1218.e4.
25. Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994. 343:1127–1130.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download