Abstract
Levofloxacin, a fluoroquinolone and L-isomer of the racemate ofloxacin, has been approved for the treatment of acute and chronic bacterial infections. Gastrointestinal complaints are the most frequently reported adverse drug reactions to fluoroquinolones. Other adverse events include headache, dizziness, increased liver enzyme levels, photosensitivity, tachycardia, QT prolongation, and eruptions. Anaphylaxis has been documented as a rare adverse drug reaction to levofloxacin; however, diagnostic tests are needed to evaluate whether these reactions are the result of levofloxacin treatment. While the results of skin tests are considered unreliable due to false-positive responses, the oral provocation test is currently considered to be the most reliable test. Tryptase, a neutral protease, is the dominant protein component of secretory granules in human mast cells, and an increased serum concentration of tryptase is a highly sensitive indicator of anaphylaxis. Herein, we report a case of levofloxacin-induced anaphylaxis in which the patient exhibited elevated serum tryptase levels and a positive oral levofloxacin challenge test result. As anaphylaxis is potentially life-threatening, the administration of fluoroquinolones to patients who have experienced a prior reaction to this type of agent should be avoided.
Levofloxacin is an L-isomer of ofloxacin that has been used to treat various infectious diseases.1 Common adverse reactions to levofloxacin include gastrointestinal problems, central nervous system disabilities, such as headache, dizziness, and insomnia, increased liver enzyme levels, QT prolongation, and skin rashes.2 Anaphylaxis is a lethal adverse event that can be caused by fluoroquinolones. It has been reported that 0.46 to 1.2 out of 100,000 individuals treated with fluoroquinolones develop anaphylaxis.3 Levofloxacin-induced anaphylaxis is rare, although there have been a few reported cases.3-5 Herein, we describe a case in which anaphylaxis was confirmed by a positive oral provocation reaction and increased serum tryptase levels.
A 58-year-old male was admitted with dyspnea. Fifteen days prior to admission, the patient had been hospitalized due to asthma exacerbations. The patient developed fever on the third day of hospitalization and was given 250 mg of levofloxacin orally. Two and a half hours later, the patient developed sudden dizziness and a skin rash, and his asthma worsened. He was treated without antibiotics and discharged with improved symptoms. However, 5 days after admittance to our hospital, his dyspnea worsened.
There were erythematous papules over the patient's entire body, and blisters were observed around his lips. The patient had visited a dermatology clinic 2 days prior. A biopsy had been performed on the patient's blisters and he was diagnosed with a herpes infection. Peripheral blood tests revealed a white blood cell count of 7,070/µL (6.0% eosinophils), a hemoglobin level of 15.5 g/dL, and a platelet count of 313,000/µL. Biochemistry tests returned the following results: AST, 146 U/L; ALT, 698 U/L; ALP, 233 U/L; gamma-GT, 868 U/L; total bilirubin, 3.1 mg/dL; and direct bilirubin level, 1.9 mg/dL. The patient's total serum immunoglobulin E (IgE) level was 150 U/mL. A chest X-ray and liver computed tomography (CT) did not reveal any abnormalities. All viral markers for hepatitis were negative. In addition, skin prick tests for 55 allergens were negative. A pulmonary function test (PFT) showed an FEV1/FVC of 59.1%, an FEV1 of 2.14 L (70%), and an FVC of 3.62 L (86%). The PC20 in a methacholine bronchial provocation test was 6.46 mg/mL.
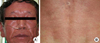
After the patient's dyspnea subsided, he was given 250 mg of levofloxacin at 10:00 AM and spirometry was performed every 30 minutes thereafter. The patient's peak expiratory flow rate (PEFR) was evaluated every hour beginning at 3 hours after oral provocation (Fig. 1). At 3.5 hours following oral provocation, a skin rash developed over the patient's entire body (Fig. 2) and he complained of dyspnea. At this time, the patient's FEV1 had decreased by 22% to 1.15 L. The patient inhaled salbutamol, and after 15 minutes his FEV1 was 1.62 L (53%). The patient complained of dizziness at 4 hours and 10 minutes after provocation; at this time, his blood pressure dropped to 67/45 mmHg. The patient was stabilized with shock positioning and a rapid saline drip. He was then given 10 mg of chlorpheniramine and 125 mg of methylprednisolone intravenously. We prepared the epinephrine before the provocation test, but mistakenly did not administer it. Two hours after the patient's blood pressure began to drop, his serum tryptase level was 17.7 µg/L (1.9-13.5 µg/L). There were no changes in PEFR during the first 13 hours and following 2 days.
Anaphylactic reactions can be classified as either IgE-mediated anaphylaxis or an IgE-independent anaphylactoid reaction.6 The presence of fluoroquinolone-specific IgE antibodies can be confirmed by radioimmunoassays. However, these assays are performed for research purposes only because it can be difficult to isolate antibodies and there is currently no standardized method available.7,8 One limitation of this report is the fact that neither a skin prick test nor serum analysis was performed. Because an IgE-mediated reaction was not excluded by a skin prick test or serum analysis, the patient was diagnosed with anaphylaxis.
To make an accurate diagnosis, additional testing methods, such as a skin prick test, intradermal test, or oral provocation test, must be utilized.9 The oral provocation test is currently the most accurate method. Although an exact mechanism has not been elucidated, studies suggest that the release of mediators such as histamine, which result from the high penetrability of fluoroquinolone into cells, plays an important role in the development of anaphylaxis.10 Thus, it has been suggested that skin prick and intradermal tests frequently produce false-positive results because fluoroquinolone can directly trigger the release of histamine.11
During an anaphylactic reaction, activated mast cells and basophils secrete histamine and tryptase. The finding of increased serum tryptase levels in patients suspected of having an anaphylactic reaction can be very useful.12 Histamine levels peak after 5 minutes and return to normal after 15-30 minutes. However, tryptase levels peak from 15-120 minutes after the onset of anaphylaxis and remain elevated for 6 hours. Therefore, these values can provide valuable diagnostic information in suspected cases of anaphylaxis.13,14 In the present case, the tryptase level became elevated 2 hours after the point at which the patient's blood pressure had decreased.
Previous studies have shown a correlation between drug allergies and viral infections.15 Fluoroquinolone administration is associated with patients infected with human immunodeficiency virus (HIV); according to Kelesidis et al.,10 10 of 22 ciprofloxacin-induced anaphylaxis patients were HIV-positive. These findings have been ascribed to the over-production of cytokines, including interferon-gamma, which expedite the activation of the immune system.16 In the present case, the patient had been diagnosed with a herpes infection following a blister biopsy. Herpes is known to be correlated with drug hypersensitivity syndrome.17 No studies have assessed the association between anaphylaxis and herpes infections; however, given that the patient in this report presented with both a herpes infection and anaphylaxis, future studies should be performed to evaluate this topic.
Figures and Tables
Fig. 1
Following the oral provocation test, the patient's FEV1 and PEFR were measured. The patient's PEFR dropped to as low as 28% of the baseline value after 3 hr. The patient's FEV1 dropped to as low as 62% of the baseline value after 3.5 hr. Normal FEV1 and PEFR values were gradually restored following treatment. Ultimately, the patient's baseline FEV1 and PEFR levels were attained.

References
1. Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs. 2003. 63:2769–2802.
2. Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999. 28:352–364.
3. Smythe MA, Cappelletty DM. Anaphylactoid reaction to levofloxacin. Pharmacotherapy. 2000. 20:1520–1523.
4. Takahama H, Tsutsumi Y, Kubota Y. Anaphylaxis due to levofloxacin. Int J Dermatol. 2005. 44:789–790.
5. Sachs B, Riegel S, Seebeck J, Beier R, Schichler D, Barger A, Merk HF, Erdmann S. Fluoroquinolone-associated anaphylaxis in spontaneous adverse drug reaction reports in Germany: differences in reporting rates between individual fluoroquinolones and occurrence after first-ever use. Drug Saf. 2006. 29:1087–1100.
6. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004. 113:832–836.
7. Aranda A, Mayorga C, Ariza A, Doña I, Rosado A, Blanca-Lopez N, Andreu I, Torres MJ. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011. 66:247–254.
8. Manfredi M, Severino M, Testi S, Macchia D, Ermini G, Pichler WJ, Campi P. Detection of specific IgE to quinolones. J Allergy Clin Immunol. 2004. 113:155–160.
9. Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The diagnosis and management of anaphylaxis. Compr Ther. 2004. 30:111–120.
10. Kelesidis T, Fleisher J, Tsiodras S. Anaphylactoid reaction considered ciprofloxacin related: a case report and literature review. Clin Ther. 2010. 32:515–526.
11. Campi P, Pichler WJ. Quinolone hypersensitivity. Curr Opin Allergy Clin Immunol. 2003. 3:275–281.
12. Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987. 316:1622–1626.
13. Brown SG, Blackman KE, Heddle RJ. Can serum mast cell tryptase help diagnose anaphylaxis? Emerg Med Australas. 2004. 16:120–124.
14. Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia. 2004. 59:695–703.
15. Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007. 33:124–133.
16. Pirmohamed M, Park BK. HIV and drug allergy. Curr Opin Allergy Clin Immunol. 2001. 1:311–316.
17. Hashimoto K, Yasukawa M, Tohyama M. Human herpesvirus 6 and drug allergy. Curr Opin Allergy Clin Immunol. 2003. 3:255–260.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download