Abstract
Interleukin 5 (IL-5) is a key cytokine involved in the induction of T-helper type 2 (Th2) responses in the asthmatic airway. We investigated IL-5 genetic polymorphisms associated with asthma phenotypes, including IgE responses to staphylococcal enterotoxins A and B (SEA and SEB, respectively), in asthmatics. Adult asthmatics (n=310) and normal controls (n=160) were enrolled in the present study. Serum total and specific IgE to SEA and SEB were measured. Two IL-5 polymorphisms, -746A>G and +4499T>G, were genotyped using the primer-extension method. There were no significant differences in genotype or haplotype frequencies of these polymorphisms between the two groups. Asthmatics carrying the AG/GG genotype at -746A>G had a significantly higher prevalence of serum specific IgE to SEA (P=0.008), higher total IgE levels (P=0.014), and lower PC20 methacholine levels (P=0.002) compared to those with the AA genotype. These findings suggest that the IL-5 promoter polymorphism at -746A>G enhances serum total and specific IgE responses to SEA, which may augment airway hyperresponsiveness in adult asthmatics.
Eosinophils are major effector cells in airway inflammation and remodeling. Interleukin 5 (IL-5) plays an important role in eosinophil activation and airway hyperresponsiveness.1 Staphylococcus aureus (SA) is a common human bacterial pathogen that produces different serological types of enterotoxins, such as Staphylococcus enterotoxins A, B, and C, (SEA, SEB, and SEC, respectively) and toxic shock syndrome toxin 1 (TSST-1), which act as superantigens. Superantigens can induce T-helper type 2 (Th2) cell-mediated immune responses and stimulate IL-5 production.2 Several studies have suggested that superantigens may be involved in the pathogenesis of respiratory allergic diseases, including asthma and rhinitis. Sensitization rates to SEA and SEB were higher in patients with asthma and/or allergic rhinitis compared to controls.3 In addition, the expression of the T cell receptor variable β-chain (Vβ8+) to which SA enterotoxins specifically bind was significantly higher in poorly controlled asthmatics than in well-controlled asthmatics and healthy controls.4 Our previous report demonstrated that levels of IgE specific to SEA in nasal polyp tissue derived from asthmatic patients closely correlated with the level of ECP and IL-5,5 indicating that IgE responses to SEA contribute to eosinophil activation in asthmatic patients.
Some reports have suggested that IL-5 polymorphisms at -746A>G and +4499T>G are significantly associated with total IgE levels and lower lung function in childhood asthma and atopic dermatitis across different ethnic populations.6-8 In the present study, we hypothesized that IL-5 genetic polymorphisms may be involved in adult asthma pathogenesis with respect to specific IgE responses to staphylococcal superantigens.
We performed a case-controlled study of 310 adult asthmatics and 160 normal controls who were enrolled from Ajou University Hospital, Suwon, South Korea. Diagnosis of asthma in all patients was conducted in accordance with the International Global Initiative for Asthma guidelines. Normal controls were recruited from healthy individuals using a questionnaire. Criteria for inclusion included no history of respiratory symptoms and normal findings on simple chest radiograms and spirometry. All subjects provided informed consent prior to participating in this study. Skin prick testing was performed with 55 common aero-allergens (Bencard Co., West Sussex, UK). Atopy was defined as one or more positive reactions to common inhalant allergens. Clinical parameters, including rhinosinusitis and pulmonary functional tests were followed as described previously.9 Serum total IgE, eosinophil cationic protein (ECP), and IgE specific to two superantigens, SEA and SEB, were measured using the UniCAP system (Phadia, Valinge, Sweden), according to the manufacturer's instructions. We considered a specific IgE level of >0.35 KU/L as positive. All PC20 methacholine, ECP, peripheral blood eosinophils, and total IgE data were log10 transformed before analysis.9
Forty healthy Korean volunteers were sequenced for polymorphisms of the IL-5 gene using an ABI Prism 3100 DNA analyzer (Applied Biosystems, Foster City, CA, USA). We selected two polymorphisms in the IL-5 gene, one in the promoter (-746A>G) and one in the 3'UTR (+4499T>G). Genomic DNA was prepared from peripheral blood samples using the Puregene DNA purification kit (Gentra, Minneapolis, MN, USA). Both polymorphisms were genotyped using the primer-extension method. Sequences of the amplification and extension primers for the -746A>G polymorphism were as follows: forward 5'-tgaacatgtgacccttgtc-3', reverse 5'-gatcctttctgttgccagt-3', and extension 5'-aagatgatgtcyagactcctggatct-3'. Sequences of the amplification and extension primers for the +4499T>G polymorphism were as follows: forward 5'-ggaggatatggtgaatgaaa-3', reverse 5'-ggtggtgcacatctgtagt-3', and extension 5'-tttacagatcatctctttgttttttt-3'.
Genotype frequency between the two groups was examined using the χ2 test. Differences in genotype and haplotype frequencies between the two groups were examined using a logistic regression analysis with co-dominant, dominant, and recessive models after accounting for age and sex as co-variables. Differences in the mean values of the phenotypic characteristics among the asthma patients, according to genotype, were compared by analysis with χ2 for categorical variables and independent t-tests for continuous variables. The haplotype block pattern was constructed using Haploview 4.2 software. P values of less than 0.05 were considered to be statistically significant.
The mean age was significantly higher in patients with asthma when compared to normal controls (42.77±14.36 vs. 30.48±10.31 years, respectively; P<0.001). Asthmatic patients exhibited significantly higher atopy rates (55.8 vs. 11.2%, respectively; P<0.001) and higher serum total IgE levels (5.08±1.44 vs. 3.55±1.35 IU/mL respectively; P<0.001) than controls.
The genotype and haplotype frequencies for the two polymorphisms in IL-5 were analyzed and compared between asthmatic patients and normal controls. The two polymorphisms were in linkage disequilibrium (/D'/=0.881, r2=0.259). In addition, the genotype and haplotype frequencies of these two polymorphisms did not differ significantly between asthmatics and normal controls based on a logistic regression analysis (Table 1).
When all of the clinical parameters were compared according to genotype, a significant association was noted between the polymorphism IL-5 -746A>G and asthma phenotypes. The asthmatic subjects with the AG/GG genotype at -746A>G had significantly lower levels of PC20 methacholine than those with the AA genotype (P=0.002, Table 2, Figure A). Moreover, asthmatic subjects carrying the AG/GG genotype at -746A>G showed significantly higher total IgE levels and a higher prevalence of specific IgE responses to SEA than those with the AA genotype (P=0.014 and P=0.008, respectively; Table 2, Figure B and C). However, IgE responses specific to SEB, house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), and other asthma related parameters, such as TEC, ECP, and the prevalence of rhinosinusitis and atopy rate, did not show any significant association (Table 2). The IL-5 genetic polymorphism at +4499T>G was not significantly associated with any of the clinical parameters tested.
We evaluated the associations of two IL-5 gene polymorphisms in asthmatics and normal controls in a Korean population. Previous studies have demonstrated that the GG genotype of IL-5 at -746A>G was associated with total IgE responses and lower lung function in Korean children with atopic asthma, and atopy status in German children.6,7 The other IL-5 polymorphism, +4499T>G, was associated with atopic dermatitis in Koreans.8 In the present study, we demonstrated a significant association of the IL-5 -746A>G polymorphism with serum total and specific IgE responses to SEA and the degree of airway hyperresponsiveness in a cohort consisting of adult Korean asthmatics.
We hypothesize that SA superantigens stimulate B lymphocytes to induce class-switching with local production of polyclonal IgE and specific IgE against bacterial superantigens. This could induce mast cells and other inflammatory cells to release variable mediators and cytokines that contribute to the development of airway inflammation in asthmatic patients.2,10 SA superantigens could also stimulate selected T-cell receptor Vβ-chains and enhance polyclonal expansion of T cells, especially Th2-driven inflammation and IL-5 production, leading to the promotion of eosinophilic inflammation.2,10 Pre-exposure to SEA in an in vivo OVA-challenge model led to elevated eosinophil numbers in bone marrow and bronchoalveolar lavage fluid, indicating that SEA exposure promotes eosinopoesis.11
Among the staphylococcal enterotoxins, SEA is the most potent, expressing two distinct binding sites for MHC class II molecules, while SEB binds to one site.12 Our previous study showed that SEA-specific IgE levels were higher in nasal polyp tissue homogenates from patients with aspirin-exacerbated respiratory disease than those with aspirin-tolerant asthma.5 In addition, significant correlations were found between ECP and specific IgE levels to staphylococcal enterotoxins, in which SEA-specific IgE was more closely correlated with ECP or IL-5 levels than with SEB-specific IgE levels. Furthermore, children with high levels of specific IgE to SEA in adenoid tissue had significantly higher levels of serum total IgE and peripheral eosinophil counts,13 suggesting that the IgE response to superantigen, particularly SEA, may be involved in eosinophil activation.
Asthmatic patients with high levels of total and specific IgE to staphylococcal superantigen present with more severe types of asthma, airway hyperresponsiveness, and poor lung function parameters.14,15 The degree of airway hyperresponsiveness is associated with late-phase responses composing eosinophil infiltration in the asthmatic airway.16 Although the prevalence of serum specific IgE to SEA or SEB was not significantly different according to severity in our cohort, adult asthmatic patients carrying the AG/GG genotype of IL-5 -746A>G had a significantly higher prevalence of serum IgE specific to SEA, higher total IgE levels, and showed more severe airway hyperresponsiveness to methacholine. Taken together, these findings suggest that IgE responses to SA superantigen increase total IgE production, eosinophil activation, and the progression of airway hyperresponsiveness in susceptible patients carrying the G allele at IL-5 -746A>G.
In conclusion, the promoter polymorphism of IL-5 at -746A>G enhances serum total and specific IgE responses to SEA, which may augment airway hyperresponsiveness to methacholine in adult asthmatic patients. Further studies are required to understand the molecular genetic mechanisms by which IL-5 polymorphisms differentially interact with SA superantigen exposure and activate eosinophils in upper and lower airway allergic inflammation.
Figures and Tables
 | FigureComparison of clinical parameters according to the genotype of the IL-5 polymorphisms in asthmatic patients A: Log PC20 methacholine levels according to the -746A>G polymorphism. B: Log total IgE level according to the -746A>G polymorphism. C: Prevalence of IgE specific to SEA according to the -746A>G polymorphism. |
Table 1
Genotype and haplotype frequencies of the IL-5 gene
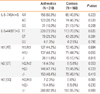
Table 2
Comparison of clinical parameters according to the genotype of the IL-5 polymorphism in asthmatic patients

ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Health 21 R&D project, Ministry of Health & Welfare, ROK (A111218-11-PG01).
References
1. Shi HZ, Xiao CQ, Zhong D, Qin SM, Liu Y, Liang GR, Xu H, Chen YQ, Long XM, Xie ZF. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am J Respir Crit Care Med. 1998. 157:204–209.
2. Barnes PJ. Intrinsic asthma: not so different from allergic asthma but driven by superantigens? Clin Exp Allergy. 2009. 39:1145–1151.
3. Lee JH, Lin YT, Yang YH, Wang LC, Chiang BL. Increased levels of serum-specific immunoglobulin E to staphylococcal enterotoxin A and B in patients with allergic rhinitis and bronchial asthma. Int Arch Allergy Immunol. 2005. 138:305–311.
4. Hauk PJ, Wenzel SE, Trumble AE, Szefler SJ, Leung DY. Increased T-cell receptor vbeta8+ T cells in bronchoalveolar lavage fluid of subjects with poorly controlled asthma: a potential role for microbial superantigens. J Allergy Clin Immunol. 1999. 104:37–45.
5. Suh YJ, Yoon SH, Sampson AP, Kim HJ, Kim SH, Nahm DH, Suh CH, Park HS. Specific immunoglobulin E for staphylococcal enterotoxins in nasal polyps from patients with aspirin-intolerant asthma. Clin Exp Allergy. 2004. 34:1270–1275.
6. Hong SJ, Lee SY, Kim HB, Kim JH, Kim BS, Choi SO, Lee SG, Shin ES, Hong TJ. IL-5 and thromboxane A2 receptor gene polymorphisms are associated with decreased pulmonary function in Korean children with atopic asthma. J Allergy Clin Immunol. 2005. 115:758–763.
7. Kabesch M, Depner M, Dahmen I, Weiland SK, Vogelberg C, Niggemann B, Lau S, Illig T, Klopp N, Wahn U, Reinhardt D, von Mutius E, Nickel R. Polymorphisms in eosinophil pathway genes, asthma and atopy. Allergy. 2007. 62:423–428.
8. Namkung JH, Lee JE, Kim E, Cho HJ, Kim S, Shin ES, Cho EY, Yang JM. IL-5 and IL-5 receptor alpha polymorphisms are associated with atopic dermatitis in Koreans. Allergy. 2007. 62:934–942.
9. Palikhe NS, Kim SH, Cho BY, Ye YM, Hur GY, Park HS. Association of three sets of high-affinity IgE receptor (FcepsilonR1) polymorphisms with aspirin-intolerant asthma. Respir Med. 2008. 102:1132–1139.
10. Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008. 225:226–243.
11. Mariano NS, de Mello GC, Ferreira T, Schenka A, Camargo EA, de Nucci G, DeSouza IA, Antunes E. Pre-exposure to Staphylococcal enterotoxin A exacerbates the pulmonary allergic eosinophil recruitment in rats. Int Immunopharmacol. 2010. 10:43–49.
12. Svensson LA, Schad EM, Sundström M, Antonsson P, Kalland T, Dohlsten M. Staphylococcal enterotoxins A, D, and E. Structure and function, including mechanism of T-cell superantigenicity. Prep Biochem Biotechnol. 1997. 27:111–141.
13. Shin SY, Choi GS, Lee KH, Kim SW, Cho JS, Park HS. IgE response to staphylococcal enterotoxins in adenoid tissues from atopic children. Laryngoscope. 2009. 119:171–175.
14. Kowalski ML, Cieślak M, Pérez-Novo CA, Makowska JS, Bachert C. Clinical and immunological determinants of severe/refractory asthma (SRA): association with Staphylococcal superantigen-specific IgE antibodies. Allergy. 2011. 66:32–38.
15. Naqvi M, Choudhry S, Tsai HJ, Thyne S, Navarro D, Nazario S, Rodriguez-Santana JR, Casal J, Torres A, Chapela R, Watson HG, Meade K, Rodriguez-Cintron W, Lenoir M, Avila PC, Burchard EG. Association between IgE levels and asthma severity among African American, Mexican, and Puerto Rican patients with asthma. J Allergy Clin Immunol. 2007. 120:137–143.
16. Diaz P, Gonzalez MC, Galleguillos FR, Ancic P, Cromwell O, Shepherd D, Durham SR, Gleich GJ, Kay AB. Leukocytes and mediators in bronchoalveolar lavage during allergen-induced late-phase asthmatic reactions. Am Rev Respir Dis. 1989. 139:1383–1389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download