Abstract
Mycoplasma pneumoniae (Mp) is a unique pathogen that causes not only pulmonary but also extrapulmonary manifestations that must be rapidly diagnosed. A 12-year-old boy, with no relevant medical history, presented with fever, severe epigastric pain, and vomiting. Laboratory findings showed fulminant and cholestatic hepatitis, hemolytic anemia, thrombocytopenia, acute kidney injury, disseminated intravascular coagulopathy, acute myocardial infarction, and rhabdomyolysis. His clinical condition rapidly deteriorated during intubation and continuous renal replacement therapy.
Despite intensive treatment, he did not recover. We report a case of fulminant and fatal multiple organ failure in a previously healthy boy with Mp infection, describing the possible pathomechanisms of multiple organ failure involved in the disease.
Mycoplasma pneumoniae (Mp) is the second most common cause of community-acquired pneumonia (CAP) in Korea,1 but is the most common pathogen in school-age children and adolescents (≥40% of CAP in this age group).2 It also affects children below five years of age and typically causes Mp pneumonia, that is usually benign and self-limited. However, Mp infection is accompanied by extrapulmonary manifestations in about 20-25% of infected children.3 Infection may present with a multitude of symptoms and signs, such as hepatic, hematological, musculoskeletal, cardiac, dermatological, gastrointestinal, neurological, and renal symptoms before, during, after or even in the absence of respiratory symptoms. Life-threatening conditions occur very rarely in previously healthy children. Here, we report a case of multiple organ failure with an unfavorable outcome associated with Mp infection, despite intensive treatment.
A 12-year-old boy who looked severely ill was transferred to our hospital due to a fever lasting for three days and intolerable epigastric pain. He had been diagnosed with unspecified pneumonia without any distinctive respiratory symptoms and treated with cefotaxime and amikacin for 14 days. On physical examination, scattered petechiae and purpura were observed on various regions of his body. Additionally, there was hepatomegaly and severe tenderness in the right upper quadrant.
Initial laboratory findings revealed leukocytosis (21,100/mm3, range 4,000-10,000) and thrombocytopenia (98,000/mm3, range 150,000-450,000). The C-reactive protein level was high at 17.50 mg/dL (range 0.0-0.8). Elevated serum creatinine (Cr, 1.7 mg/dL), oliguria (240 mL) for more than 24 hours, and urine spot analysis with low sodium excretion fraction (0.62) revealed acute kidney injury. There was also acute hepatic failure, as indicated by markedly high aspartate aminotransferase (AST, 2,023 U/L; range 5-40), alanine aminotransferase (ALT, 482 U/L; range 8-40), and γ-glutamyl transferase (106 IU/L; range 10-75 IU/L). Prothrombin time (PT) was 24.9 seconds (INR 2.12) and activated partial thromboplastin time (aPTT) 123 seconds. Serum creatinine kinase (CK) was 5,248 mg/dL (range 30-180), lactate dehydrogenase 8,740 U/L (range 100-200), serum myoglobin 273.6 ng/mL (range 0.0-72.0), and urine myoglobin 2,927 ng/mL (range 0.0-30), suggesting severe rhabdomyolysis.
Twelve hours after admission, serial laboratory testing indicated rapidly deteriorating multiple organ failure (Table). Liver function results were serum AST, 33,221 U/L; ALT, 11,096 U/L; total bilirubin, 9.9 mg/dL (range 0.2-1.2); direct bilirubin, 4.0 mg/dL (range 0.0-0.4); and ammonia (NH3) 117 µmol/L (range 0.0-54). PT and aPTT were markedly prolonged over 200 seconds. Serum Cr and uric acid concentrations had also increased to 2.9 mg/dL and 10.3 mg/dL, respectively. Serum electrolyte levels showed hyponatremia (Na+ 129 mMol/L), hyperkalemia (K+ 7.1 mMol/L) and severe metabolic acidosis (tCO2 8 mMol/L). D-dimer, fibrinogen degradation product and antithrombin III were 37,492 ng/mL (range 0.0-200), above 20 ng/mL (range 0.0-4.0), and 48% (range 65-125), respectively. The patient was stuporous, and we decided to intubate and to apply continuous renal replacement therapy (CRRT) using veno-venous hemodiafiltration to remove all toxins such as myoglobin and proinflammatory cytokines, and to restore the functions of failed organs.
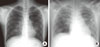
On hospital day 3, pleural effusion was found on a chest radiogram, and thoracostomy was performed (Figure). Viral markers for hepatitis, blood cultures, sputum cultures, and autoantibodies were all negative. However, over two weeks, the titer of anti-mycoplasma IgM and IgG antibodies increased from below 1:40 to more than 1:1,280 IU/mL. Intravenous clarithromycin had been administered from admission day due to suspicion of Mp. An electroencephalogram showed the severe isoelectric pattern of diffuse encephalopathy, suggesting a critical prognosis. The electrocardiography recorded ventricular tachycardia and precordial ST segment elevation, both signs of acute myocardiac infarction, with elevated CK-MB enzymes of 278.2 µg/L (range 0.0-5.0). The patient went into cardiac arrest and did not recover.
Over the last 20 years, unusual and extrapulmonary manifestations of the Mp infection have been reported only infrequently.3,4 Most reported cases showed favorable outcomes, and fulminant and fatal multiple organ involvement, with more than nine extrapulmonary manifestations simultaneously (fulminant and cholestatic hepatitis, hemolytic anemia, thrombocytopenic purpura, rhabdomyolysis, pleural effusion, acute kidney injury, disseminated intravascular coagulopathy, myocarditis, and acute myocardial infarction), as seen in our patient, was extremely rare.
The exact pathomechanism of the Mp infection-induced multiple organ failure remains unclear. It has been postulated that the cytadherence of Mp, cell invasion, cytotoxicity, immune response, and cytokine production might be involved in its pathogenicity.5,6 Recently, Narita7 suggested a plausible pathogenesis of the extrapulmonary manifestations of Mp infection, classifying them into three categories: Firstly, a direct-type, associated with inflammatory cytokines induced locally by lipoproteins contained in the bacterial cell membrane. Secondly, an indirect-type, associated with immune modulation, such as autoimmunity, allergy, or immune complexes. Thirdly, a vascular occlusion-type, associated with vasculitis and/or thrombosis, with or without a systemic hypercoagulable state induced by the bacterium. The production of various pro-inflammatory cytokines, such as tumor necrosis factor-α, interferon-γ, and interleukin-8, may occur in all three types. Gehrs et al.8 reported that hemolytic anemia in Mp infection was mediated by the Mp-associated cold antibody (IgM), and that cold agglutinins developed during the second and third week of illness. The toxicity of Mp infection is prominent in the late stages of the illness.9
In the majority of children, Mp-associated hepatitis is usually self-limited, but fulminant hepatitis with unfavorable outcome can develop, as seen in the case presented here. Berg et al.10 found that Mycoplasma antigens can trigger the induction of anti-mitochondrial antibodies, especially through MpPDC-E2-related antibodies and immune reactivity. Carrascosa et al.11 also reported a case of lethal hepatitis associated with hepatotoxic drug treatment in a patient with acute Mp infection.
In this case, CRRT was started early, but was not successful. However, CRRT is recommended as the treatment of choice when rhabdomyolysis coexists with acute kidney injury. Serial serologic testing of Mp infection is necessary for early diagnosis and appropriate macrolide treatment, which is mandatory in order to rescue patients with extrapulmonary manifestations. Corticosteroids can also be used in combination with macrolide antibiotics, for supplementary anti-inflammatory effects.12 However, our patient did not recover, despite CRRT and corticosteroid treatment.
In conclusion, fulminant and fatal multiple organ failure might be associated with Mp infection in children through various pro-inflammatory cytokines. Even in cases with no respiratory symptoms, we emphasize the necessity of awareness of the variety and severity of Mp infection in children presenting with extrapulmonary manifestations. Additionally, an accumulation of clinical reports is important for distinguishing what Mp can and cannot cause, on the basis of biological ability, because Mycoplasma infections are strictly species-specific.7
Figures and Tables
References
1. Chong YP, Jung KS, Lee KH, Kim MN, Moon SM, Park S, Hur J, Kim DM, Jeon MH, Woo JH. The bacterial etiology of community-acquired pneumonia in Korea: A nationwide prospective multicenter study. Infect Chemother. 2010. 42:397–403.
2. Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008. 56:326–331.
3. Kottayam R, Rozenberg G, Cohn RJ. Unusual haematologic manifestations of Mycoplasma pneumoniae infection. J Paediatr Child Health. 2007. 43:80–82.
4. Timitilli A, Di Rocco M, Nattero G, Tacchella A, Giacchino R. Unusual manifestations of infections due to Mycoplasma pneumoniae in children. Infez Med. 2004. 12:113–117.
5. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008. 32:956–973.
6. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004. 17:697–728.
7. Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010. 16:162–169.
8. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002. 69:258–271.
9. Smith LG. Mycoplasma pneumonia and its complications. Infect Dis Clin North Am. 2010. 24:57–60.
10. Berg CP, Kannan TR, Klein R, Gregor M, Baseman JB, Wesselborg S, Lauber K, Stein GM. Mycoplasma antigens as a possible trigger for the induction of antimitochondrial antibodies in primary biliary cirrhosis. Liver Int. 2009. 29:797–809.
11. Carrascosa MF, Lucena MI, Andrade RJ, Caviedes JR, Lavín AC, Mones JC, Rivero AP, Serrano VB. Fatal acute hepatitis after sequential treatment with levofloxacin, doxycycline, and naproxen in a patient presenting with acute Mycoplasma pneumoniae infection. Clin Ther. 2009. 31:1014–1019.
12. Tagliabue C, Salvatore CM, Techasaensiri C, Mejias A, Torres JP, Katz K, Gomez AM, Esposito S, Principi N, Hardy RD. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J Infect Dis. 2008. 198:1180–1188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download