Abstract
Purpose
Bronchial hyperresponsiveness (BHR) is typically measured by bronchial challenge tests that employ direct stimulation by methacholine or indirect stimulation by adenosine 5'-monophosphate (AMP). Some studies have shown that the AMP challenge test provides a better reflection of airway inflammation, but few studies have examined the relationship between the AMP and methacholine challenge tests in children with asthma. We investigated the relationship between AMP and methacholine testing in children and adolescents with atopic asthma.
Methods
The medical records of 130 children with atopic asthma (mean age, 10.63 years) were reviewed retrospectively. Methacholine and AMP test results, spirometry, skin prick test results, and blood tests for inflammatory markers (total IgE, eosinophils [total count, percent of white blood cells]) were analyzed.
Results
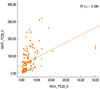
The concentration of AMP that induces a 20% decline in forced expiratory volume in 1 second [FEV1] (PC20) of methacholine correlated with the PC20 of AMP (r2=0.189, P<0.001). No significant differences were observed in the levels of inflammatory markers (total eosinophil count, eosinophil percentage, and total IgE) between groups that were positive and negative for BHR to methacholine. However, significant differences in inflammatory markers were observed in groups that were positive and negative for BHR to AMP (log total eosinophil count, P=0.023; log total IgE, P=0.020, eosinophil percentage, P<0.001). In contrast, body mass index (BMI) was significantly different in the methacholine positive and negative groups (P=0.027), but not in the AMP positive and negative groups (P=0.62). The PC20 of methacholine correlated with FEV1, FEV1/forced vital capacity (FVC), and maximum mid-expiratory flow (MMEF) (P=0.001, 0.011, 0.001, respectively), and the PC20 of AMP correlated with FEV1, FEV1/FVC, and MMEF (P=0.008, 0.046, 0.001, respectively).
Bronchial hyperresponsiveness (BHR) is an exaggerated bronchoconstrictive response of the airway to diverse stimuli and is a defining characteristic of asthma.1 BHR is usually measured by a direct stimulation test that employs methacholine or an indirect stimulation test that employs adenosine 5'-monophosphate (AMP). Direct stimulation of the airway by methacholine leads to bronchoconstriction via the activation of cholinergic receptors in bronchial smooth muscle. Indirect stimulation of the airway by AMP leads to bronchoconstriction through degranulation of mast cells and the release of inflammatory mediators such as histamine and leukotrienes.2
Previous studies have shown that inhaled corticosteroid use improves the concentration of AMP that induces a 20% decline in forced expiratory volume in 1 second (FEV1) (PC20) more so than the PC20 of methacholine. Additionally, an improved BHR after remaining in a hypoallergenic environment for 1 month can be detected by AMP testing, but not by methacholine testing.3 These findings suggest that AMP testing provides a more sensitive measure of airway inflammation than that of methacholine testing.
Several studies have compared the relationships between BHR and AMP or methacholine in children with atopic and non-atopic asthma, but the results have been inconsistent.2,4-7 A recent population-based study showed that assessing bronchial responsiveness with AMP testing provided a better measure of the atopic status of children with asthma.8-10 However, only a few reports have compared AMP and methacholine responsiveness in children with atopic asthma.11
In the present study, we investigated the use of methacholine and AMP testing in children with atopic asthma.
The medical records of 130 children (mean age, 10.63 years) with mild intermittent atopic asthma who were given methacholine and AMP challenge tests were reviewed retrospectively. All 130 children had histories of recurrent wheezing and cough, favorable responses to inhaled corticosteroids and/or inhaled bronchodilators, and had been diagnosed with asthma. This study was performed at the Childhood Asthma and Atopy Center of Asan Medical Center Children's Hospital from May 2009 to May 2011. The study protocol was approved by the Institutional Review Board of Asan Medical Center, and all parents and guardians provided written informed consent following a detailed explanation of the study.
Bronchial challenge testing with AMP followed by methacholine was performed in all recruited children. Methacholine was administered immediately after recovery of pulmonary function. None of the subjects showed symptoms of severe bronchial narrowing; thus, no bronchodilators were administered after the challenge tests. Subjects did not use any medications for asthma at least 2 months before the bronchial challenge test. A pulmonary function test with a bronchodilator was performed once after bronchial challenge testing using methacholine. Basal lung function, including forced vital capacity (FVC), FEV1, peak expiratory flow, and forced expiratory flow at the mid-portion of FVC were also measured. FVC and FEV1 were measured each time when there was a concentration change in the methacholine and AMP tests. Fresh solutions of methacholine and AMP were prepared in buffered saline solution at concentrations of 0.625, 1.25, 2.5, 5, 10, and 25 mg/mL for methacholine and 3.125, 6.25, 12.5, 50, 100, 200, and 400 mg/mL for AMP. The methacholine PC20 and AMP PC20 were calculated by interpolation between adjacent data points when the FEV1 decreased >20%. Censored values of 50 mg/mL for the methacholine PC20 and 800 mg/mL for AMP were given to children who did not have 20% declines in FEV1 after inhaling maximal concentrations of methacholine (25 mg/mL) or AMP (400 mg/mL). Children were considered to have BHR when the methacholine PC20 was <8 mg/mL12,13 and the AMP PC20 was <200 mg/mL.
Skin-prick testing was performed on the backs of children using standard methods. Commercial extracts of the following common allergens were used: mites (Dermatophagoides pteronyssinus and D. farina), molds (Alternaria, Aspergillus, Cladosporium, and Penicillium), pollens (grasses, trees, weed, ragweed, mugwort, oak, beech, nettle, willow, elm, pine, hop, elder, hazel, oats, lambs quarter, ash, alder, birch, timothy, and rye grass), foods (apple, beef, chicken, egg white, codfish, crab, lobster, milk, mushroom, oyster, peach, peanut, pork, mussel, shrimp, strawberry fruit, tomato, tuna, walnut, and wheat flour), dog and cat epithelia, and cockroach. Histamine and isotonic saline were used as positive and negative controls, respectively. A wheal diameter that was greater than the positive control was considered a positive response when the positive control was greater than 3 mm. Any child with a response to one or more allergens was considered atopic.
Total eosinophil counts and the percentage of blood eosinophils were measured with an automated blood analyzer. Serum total IgE was measured by a fluorescence enzyme immunoassay using the ImmunoCAP system (Phadia AB, Uppsala, Sweden).
The levels of IgE and eosinophil counts were log-transformed before analyses to normalize the distributions. The values or frequencies between the two groups were compared with a Student's t-test. Pearson's correlation test was used to evaluate the relationship between the PC20 of methacholine and that of AMP with values from the pulmonary function test (PFT) and inflammatory markers. A P value ≤0.05 was considered statistically significant. SPSS ver.18 was used for the analysis (Chicago, IL, USA).
In total, 130 children who were given skin-prick testing, methacholine challenge testing, and AMP challenge testing were enrolled. All children had positive reactions to at least one of the allergens in the skin-prick test and, thus, were considered to have atopy. Table 1 shows the baseline characteristics of the enrolled children. More boys (71.5%) were included than girls (28.5%), and the mean age of the participants was 10.63±3.50 years (range, 6-18 years). A total of 30% of the children had family histories of allergic disease, 70% had allergic rhinitis, and 23.8% had atopic dermatitis (Table 1). Approximately 86% (111/130) of patients were positive for the methacholine challenge, and 93.8% (114/122) of patients were positive for the AMP challenge (Table 3).
The methacholine PC20 and AMP PC20 values were weakly correlated with each other (r2=0.189, P<0.001, Figure). Furthermore, the PC20 values for methacholine and AMP were both correlated with the PFT parameters (Table 2). In particular, FEV1, FEV1/FVC%, and maximum mid-expiratory flow (MMEF) were significantly correlated with the PC20 of methacholine and AMP (methacholine: P=0.001, 0.011, and 0.001, respectively; AMP: P=0.008, 0.046, and 0.001, respectively).
Table 3 shows no differences in the levels of three inflammatory markers in the group with positive BHR to methacholine (PC20<8 mg/mL) or the group with negative BHR to methacholine (PC20≥8 mg/mL). In contrast, significant differences were observed between the group with positive BHR to AMP (PC20<200 mg/mL) and the group with negative BHR to AMP (PC20≥200 mg/mL) for log total eosinophil count, percentage of eosinophils, and log IgE (P=0.023,<0.001, and 0.020, respectively) (Table 3). The co-occurrence of allergic rhinitis or atopic dermatitis was not significantly correlated with the PC20 of methacholine or AMP (allergic rhinitis with methacholine PC20: P=0.905; with AMP PC20: P=0.909, atopic dermatitis with methacholine PC20: P=0.404; with AMP PC20: P=0.238 respectively, data not shown). The sex distribution did not make a significant difference in inflammatory markers between the groups with positive or negative BHR to AMP or methacholine. In contrast, body mass index (BMI) was significantly higher in the group with positive BHR to methacholine than that in the group with negative BHR to methacholine (P=0.027); however, BMI was not significantly different in the group with positive BHR to AMP and the group with negative BHR to AMP (P=0.62, Table 3).
The present study showed that BHR to methacholine and AMP are correlated with each other and with FEV1. Our results also indicated that BHR to AMP implicates airway inflammation more than that of BHR to methacholine. In contrast, BMI correlated with BHR to methacholine but not with BHR to AMP. Together, these findings suggest that the AMP challenge test provides a better estimate of airway inflammation, and that the methacholine challenge test provides a better measure of airway mechanics.
Several studies have reported that responsiveness to methacholine and AMP are indicators of BHR, but there are discrepancies in the reported prevalence rates of responsiveness to these tests in patients with atopic and non-atopic asthma.5,14 However, a recent study of 93 children with recurrent wheezing reported similar BHR to methacholine in children with atopic and non-atopic disease.6 Several explanations are possible for the discrepancies in these previous studies. Younger children tend to have smaller airways, and are thus more sensitive to airway stimulation and have a higher prevalence of hyper-responsiveness to methacholine even if they are non-atopic. Thus, we compared the BHR of methacholine and AMP in children with atopic asthma.
The BHR to AMP but not methacholine was significantly correlated with inflammatory markers (total eosinophil count, IgE level, and percentage of eosinophils), which is consistent with a previous study of 47 children with asthma that showed no significant correlation between the PC20 of methacholine and serum IgE levels.15 In contrast, several studies have reported positive correlations between the PC20 of AMP and inflammatory markers.3,9,10,16,17 Additionally, a study of 120 patients with asthma reported that an improvement in the AMP PC20 after steroid therapy is more closely associated with a reduction in airway inflammation,18 suggesting that BHR to the AMP challenge test may better represent airway inflammation as compared with BHR to the methacholine challenge test.
Previous studies have reported that BHR to AMP varies from 39.4%19 to 89%.3 The methacholine challenge test is thought to be a highly sensitive method for detecting asthma.16 One study of 77 children with asthma reported that the positive response to methacholine challenge (96.1%) was greater than that of AMP challenge (85.7%).20 This is consistent with results for adults.21 However, we found higher responsiveness to the AMP challenge (94.5%) than that of the methacholine challenge (86.7%). Although the reasons for these discrepant results are unclear, it is likely that they are due to differences in study populations, such as age, sex, atopic status, and asthma severity. In particular, our study population consisted entirely of children with mild intermittent atopic asthma. This result is supported by previous studies reporting that patients with atopic asthma are significantly more responsive to AMP than to methacholine.9 Thus, although the methacholine challenge test might be sensitive for detecting asthma, it may not be useful for defining allergic inflammation of the airway or the effectiveness of steroid treatment.
Differences between the results of the methacholine and AMP challenge tests are probably due to differences in the mechanisms of these tests. Methacholine acts through a cholinergic receptor as a stimulus (direct stimulation), making the results less correlated with mechanisms of atopy. However, AMP acts by releasing inflammatory mediators; therefore, results are more correlated with atopy. It is generally accepted that airway inflammation contributes to the presence and severity of BHR.22,23 Airway inflammation and eosinophilia are key features of asthma, and an increase in eosinophils in the peripheral blood is associated with asthma severity, and is thus used as a marker of disease activity.20 IgE also has a pivotal role in asthma.24 In the present study, we observed significant differences in eosinophil percentages and counts and IgE levels between the groups with positive and negative BHR to AMP, but not in response to the methacholine test.
Additionally, BMI was significantly correlated with BHR to methacholine. Previous studies have also reported a relationship between obesity and asthma,25,26 although the underlying mechanism is unclear. Possible explanations include the following: obesity may have a mechanical effect on lung function, adipocytes may be associated with chronic low-grade inflammation, and obesity comorbidities may disrupt lung function.27 However, the link between BMI and the differences between BHR to methacholine and BHR to AMP is unclear. A previous review of 30 adult females in France reported a significant correlation between BMI and BHR to methacholine, but not to AMP.28 These results are compatible with our finding that BMI may be linked to BHR to methacholine but not BHR to AMP.
The present study had several limitations. First, this study was based on a relatively small population because we only included children with atopic asthma. Second, we did not directly measure airway inflammation biomarkers, such as sputum eosinophils or exhaled nitric oxide from the airway. However, because children with asthma include those with atopic asthma, our study showed that indirect stimulation with the AMP challenge test could be as useful as a methacholine challenge test for assessing airway hyperresponsiveness in children with atopic asthma. Furthermore, the BHR to methacholine decreases with age in children with asthma, so the AMP challenge test appears to be an effective method for measuring airway hyperresponsiveness in children and adolescents with atopic asthma. Additionally, the AMP challenge test, which reflects airway inflammation, could be a valuable method to monitor the effectiveness of steroid treatment.
In summary, our results in children with atopic asthma indicate that airway inflammation is better correlated with BHR to AMP than to methacholine. Thus, the AMP challenge test, which implicates airway inflammation, could be a useful method for diagnosing asthma. We suggest future investigations to examine BHR after steroid treatment in children with atopic asthma.
Figures and Tables
Figure
Pearson's correlation analysis of the concentration of AMP that induces a 20% decline in forced expiratory volume in 1 sec (PC20) of methacholine and the PC20 of AMP (r2=0.189, P<0.001).
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, Republic of Korea (A092076).
References
1. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006. 118:551–559. quiz 560-1.
2. Ribeiro M, Pereira CA, Nery LE, Beppu OS, Silva CO. Methacholine vs adenosine on intra and extrathoracic airway hyperresponsiveness in patients with cough variant asthma. Allergy. 2008. 63:527–532.
3. Van Den Berge M, Meijer RJ, Kerstjens HA, de Reus DM, Koëter GH, Kauffman HF, Postma DS. PC(20) adenosine 5'-monophosphate is more closely associated with airway inflammation in asthma than PC(20) methacholine. Am J Respir Crit Care Med. 2001. 163:1546–1550.
4. Bakirtas A, Turktas I. Methacholine and adenosine 5'-monophosphate challenges in preschool children with cough-variant and classic asthma. Pediatr Pulmonol. 2007. 42:973–979.
5. Castro-Rodriguez JA, Navarrete-Contreras P, Holmgren L, Sanchez I, Caussade S. Bronchial hyperreactivity to methacholine in atopic versus nonatopic asthmatic schoolchildren and preschoolers. J Asthma. 2010. 47:929–934.
6. Kim DK, Choi SH, Yu J, Yoo Y, Koh YY. Bronchial responsiveness to methacholine and adenosine 5'-monophosphate in atopic and non-atopic preschool children with recurrent wheezing. Clin Exp Allergy. 2007. 37:15–21.
7. Michils A, Elkrim Y, Haccuria A, Van Muylem A. Adenosine 5'-monophosphate challenge elicits a more peripheral airway response than methacholine challenge. J Appl Physiol. 2011. 110:1241–1247.
8. Fowler SJ, Lipworth BJ. Relationship of skin-prick reactivity to aeroallergens and hyperresponsiveness to challenges with methacholine and adenosine monophosphate. Allergy. 2003. 58:46–52.
9. Lúdvíksdóttir D, Janson C, Björnsson E, Stålenheim G, Boman G, Hedenström H, Venge P, Gudbjörnsson B, Valtysdóttir S. BHR Study Group. Different airway responsiveness profiles in atopic asthma, nonatopic asthma, and Sjogren's syndrome. BHR Study Group. Bronchial hyperresponsiveness. Allergy. 2000. 55:259–265.
10. Suh DI, Lee JK, Kim CK, Koh YY. Methacholine and adenosine 5'-monophosphate (AMP) responsiveness, and the presence and degree of atopy in children with asthma. Pediatr Allergy Immunol. 2011. 22:e101–e106.
11. Suh DI, Lee JK, Kim CK, Koh YY. Bronchial hyperresponsiveness to methacholine and adenosine 5'-monophosphate, and the presence and degree of atopy in young children with asthma. Clin Exp Allergy. 2011. 41:338–345.
12. Springer C, Godfrey S, Picard E, Uwyyed K, Rotschild M, Hananya S, Noviski N, Avital A. Efficacy and safety of methacholine bronchial challenge performed by auscultation in young asthmatic children. Am J Respir Crit Care Med. 2000. 162:857–860.
13. Yong SC, Smith CM, Wach R, Kurian M, Primhak RA. Methacholine challenge in preschool children: methacholine-induced wheeze versus transcutaneous oximetry. Eur Respir J. 1999. 14:1175–1178.
14. Kurukulaaratchy RJ, Fenn M, Matthews S, Arshad SH. Characterisation of atopic and non-atopic wheeze in 10 year old children. Thorax. 2004. 59:563–568.
15. Takeda K, Shibasaki M, Takita H. Relation between bronchial responsiveness to methacholine and levels of IgE antibody against Dermatophagoides farinae and serum IgE in asthmatic children. Clin Exp Allergy. 1993. 23:450–454.
16. Cockcroft D, Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009. 103:363–369. quiz 369-72, 400.
17. Yoo Y, Kim DK, Yu J, Choi SH, Kim CK, Koh YY. Relationships of methacholine and AMP responsiveness with peak expiratory flow variability in children with asthma. Clin Exp Allergy. 2007. 37:1158–1164.
18. van den Berge M, Kerstjens HA, Meijer RJ, de Reus DM, Koëter GH, Kauffman HF, Postma DS. Corticosteroid-induced improvement in the PC20 of adenosine monophosphate is more closely associated with reduction in airway inflammation than improvement in the PC20 of methacholine. Am J Respir Crit Care Med. 2001. 164:1127–1132.
19. Bakirtas A, Turktas I. Determinants of airway responsiveness to adenosine 5'-monophosphate in school-age children with asthma. Pediatr Pulmonol. 2006. 41:515–521.
20. Choi SH, Kim DK, Yu J, Yoo Y, Koh YY. Bronchial responsiveness to methacholine and adenosine 5'-monophosphate in young children with asthma: their relationship with blood eosinophils and serum eosinophil cationic protein. Allergy. 2007. 62:1119–1124.
21. Fowler SJ, Dempsey OJ, Sims EJ, Lipworth BJ. Screening for bronchial hyperresponsiveness using methacholine and adenosine monophosphate. Relationship to asthma severity and beta(2)-receptor genotype. Am J Respir Crit Care Med. 2000. 162:1318–1322.
22. Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010. 138:4S–10S.
23. Holgate ST, Beasley R, Twentyman OP. The pathogenesis and significance of bronchial hyper-responsiveness in airways disease. Clin Sci (Lond). 1987. 73:561–572.
24. Prenner BM. Asthma 2008: targeting immunoglobulin E to achieve disease control. J Asthma. 2008. 45:429–436.
25. Kim KM, Kim SS, Kwon JW, Jung JW, Kim TW, Lee SH, Min KU, Kim YY, Cho SH. Association between subcutaneous abdominal fat and airway hyperresponsiveness. Allergy Asthma Proc. 2011. 32:68–73.
26. Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu JH, Kim BJ, Hong SJ. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int Arch Allergy Immunol. 2011. 154:42–48.
27. Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008. 121:1087–1093. quiz 1094-5.
28. Engbers M, Vachier I, Sterk P, Bourdin A, Gras D, Godard P, Chanez P. Mild asthma in overweight women: A new phenotype? Respir Med. 2010. 104:1138–1144.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download