Abstract
Purpose
Invariant natural killer T (iNKT) cells may play an important role in the pathogenesis of asthma in mice and humans. Thus, an agent that modulates the function of iNKT cells may have therapeutic potential to control asthma. We hypothesized that lipopolysaccharide (LPS)-, flagellin-, or CpG-induced changes in the cytokine milieu may modify and even inhibit the function of airway iNKT cells in asthma.
Methods
Because increased α-galactosylceramide (GalCer)-induced airway hyperreactivity (AHR) reflects the presence of airway iNKT cells, α-GalCer-induced AHR, as well as inflammatory cells and cytokines in bronchoalveolar lavage (BAL) fluid, were determined 24 hours after in vivo treatment with LPS, flagellin, or CpG in naïve BALB/c mice. Intracellular IL-4 and IFN-γ were measured in spleen iNKT cells after in vitro treatment with LPS, flagellin, or CpG. A role for IL-12 following the treatments was determined.
Results
Intranasal administration of LPS, flagellin, or CpG reduced development of α-GalCer-induced AHR, eosinophilic airway inflammation, and Th1 and Th2 cytokine responses in BAL fluid, while producing IL-12 in BAL fluid. Intraperitoneal administration of IL-12 mAb blocked the suppressive effect of LPS, flagellin, or CpG. In vitro treatment with LPS, flagellin, or CpG reduced production of IL-4 and IFN-γ from α-GalCer-stimulated spleen iNKT cells; these effects were ameliorated by addition of anti-IL-12 mAb.
Asthma is characterized by airway hyperreactivity (AHR), eosinophilic airway inflammation, and the Th2 immune response. Natural killer T (NKT) cells are unique CD1d-restricted T cells with surface markers of NK cells, and secrete very large amounts of Th1 or Th2 cytokines immediately following activation.1 Invariant natural killer T (iNKT) cells have been reported to play an essential role in development of allergen-induced,2 α-galactosylceramide (α-GalCer)-induced,3 or ozone-induced4 AHR in experimental mice. Non-invariant NKT cells may also play a role in development of allergen-induced AHR in a mouse model of asthma.5
NKT cells may be increased in the airways of patients with asthma,6 although this is not always the case.7 We showed an inverse association between blood NKT cells and atopic indexes in stable asthmatics.8 We found that sputum NKT cells were associated with development of eosinophilic airway inflammation.9 Recently, we reported that NKT cells may play an important role in the pathogenesis of acute exacerbation in human asthma.10 NKT cells likely play an important role in the immune pathogenesis of human asthma.
If NKT cells do indeed play an important role in the pathogenesis of asthma in mice and humans, an agent capable of modulating the function of NKT cells may have therapeutic potential to control asthma. α-GalCer-activated iNKT cells have been reported to prevent development of eosinophilic airway inflammation via IFN-γ production.11 Alternatively, a therapeutic to alter cytokine expression profiles of NKT cells might be considered. In the past several decades, the incidence of asthma and allergic diseases has increased, particularly in developed countries,12 which may be partly explained by the hygiene hypothesis.13,14 The reduction of infectious diseases may promote development of asthma and atopic diseases. Indeed, treatment with lipopolysaccharide (LPS),15 flagellin,16 or CpG17 has been reported to inhibit development of asthma, suggesting that these Toll-like receptor (TLR) agonists might suppress the functions of iNKT cells. The production of IL-12 from antigen-presenting cells can be induced by exposure of the cells to LPS,18 flagellin,19 or CpG.20 Thus, LPS-, flagellin-, or CpG-induced changes in the microenvironmental cytokine milieu may modify the function of iNKT cells in asthma via IFN-γ production. Additionally, anergy or hyporesponsiveness of iNKT cells can result following activation by α-GalCer 21-23 or bacteria.19 IL-12 might play a role in induction of iNKT cell anergy following microorganism-induced iNKT cell activation,19 which might be a therapeutic modality to induce iNKT cell tolerance in asthma.
In the present study, we investigated whether LPS, flagellin, or CpG can inhibit the function of airway iNKT cells using a mouse model of α-GalCer-induced AHR. Intranasal administration of α-GalCer has been used to demonstrate the presence of iNKT cells in the lungs and airways, showing increased AHR 24 hours post-administration.3 Additionally, using mouse spleen iNKT cells, we determined the suppressive effects of LPS, flagellin, and CpG on spleen iNKT cells. The results are consistent with the proposal that TLR4, 5, and 9 agonists suppress the function of airway and spleen iNKT cells via IL-12-dependent mechanisms.
Female, six-week-old, BALB/c mice were purchased from Orient Bio (Seongnam, South Korea). Mice were bred in a laminar flow holding unit and housed in autoclaved cages on autoclaved bedding. Chonnam National University Medical School Committee on Animal Welfare approved the animal protocols.
Naïve mice were intranasally administered 1 µg α-GalCer (Enzo Life Sciences, Lausen, Switzerland) or PBS. AHR to methacholine was measured using a model OCP-3000 barometric plethysmographic chamber (Allmedicus, Anyang, South Korea) 24 hours after α-GalCer administration, as described previously.24
Immediately after the AHR measurements, BAL was evaluated as described previously.24 Total cells, macrophages, lymphocytes, eosinophils, and neutrophils were counted. Supernatants were kept for cytokine assays.
Spleens were removed from naïve mice. Spleen cells were incubated in RPMI 1640 media (WelGENE, Daegu, South Korea) for 16 hours, and then stimulated with 0.1 µg/mL α-GalCer. After 90 minutes, intracellular cytokines of iNKT cells were stained.
Spleen iNKT cells were identified by staining with peridinin-chlorophyll protein-conjugated CD3 mAb (BD Biosciences, San Jose, CA, USA) and phycoerythrin-conjugated CD1d tetramers (Pro Immune, Sarasota, FL, USA) loaded with α-GalCer. Unloaded CD1d tetramers were used as a control. For intracellular staining, fixation and permeabilization were performed using Cytofix/Cytoperm kits (BD Biosciences), according to the manufacturer's instructions. Cells were incubated with allophycocyanin-conjugated IL-4 (BD Biosciences) or IFN-γ mAbs (BD Biosciences), and the respective isotype IgG1 antibodies (BD Biosciences). Flow cytometry was performed using a four-color BD FACS Calibur instrument (BD Biosciences) and analyzed with FlowJo 8.7.1 software (Tree Star, Ashland, OR, USA).
In a model of α-GalCer-induced AHR, 0.1 µg LPS (Sigma-Aldrich), 10 µg flagellin, or 50 µg of CpG oligodeoxynucleotide (InvivoGen, San Diego, CA, USA) was intranasally administered 24 hours before α-GalCer administration. In our preliminary study, intranasal treatment with 0.1 µg LPS seemed to inhibit development of α-GalCer-induced AHR and airway inflammation more effectively than 3 or 100 µg LPS. In spleen cultures, LPS (0.5 ng/mL), flagellin (0.1 µg/mL), or CpG (0.1 µg/mL) was added 16 hours before α-GalCer stimulation. Vibrio vulnificus-derived recombinant flagellin B (FlaB) was used, which was cloned and purified as described previously.16
IL-12p70 mAb (100 µg; BD Biosciences) or isotype IgG2b (BD Biosciences) was administered intraperitoneally immediately before treatment with LPS, FlaB, or CpG in a model of α-GalCer-induced AHR. In spleen cultures, IL-12p70 mAb (10 µg/mL) was added immediately before treatment with LPS, FlaB, or CpG.
IL-4, IL-12, IL-13, and IFN-γ levels in BAL fluid were measured by ELISA (R&D Systems, Minneapolis, MN, USA), as described previously.24
Intranasal treatment with LPS 24 hours before α-GalCer administration inhibited development of α-GalCer-induced AHR. Treatment with LPS alone did not induce AHR. Administration of α-GalCer increased numbers of total cells, as well as of macrophages, eosinophils, neutrophils, and lymphocytes, all of which were decreased by LPS treatment. Treatment with LPS alone slightly increased inflammatory cell numbers. α-GalCer administration enhanced production of both IL-13 and IFN-γ, which were inhibited by LPS treatment. Treatment with LPS alone resulted in no production of IL-13 or IFN-γ. Administration of LPS, but not α-GalCer, induced IL-12 production (Fig. 1). Thus, these findings indicate that intranasal LPS treatment may enhance IL-12 production, and then suppress the function of iNKT cells in the airways.
Intranasal treatment with FlaB 24 hours before α-GalCer administration decreased α-GalCer-induced AHR and inflammatory cells. Treatment with FlaB decreased both IL-4 and IFN-γ, but enhanced IL-12, production (Fig. 2). These data indicate that intranasal FlaB treatment enhances IL-12 production, and then suppresses the function of iNKT cells in the airways.
Intranasal treatment with CpG 24 hours before α-GalCer administration decreased α-GalCer-induced AHR and inflammatory cell numbers. Treatment with CpG decreased both IL-4 and IFN-γ, but enhanced IL-12, production (Fig. 3). The results indicate that intranasal CpG treatment may enhance IL-12 production and then suppress the function of iNKT cells in the airways.
Injection of IL12 mAb, but not the IgG2b isotype, reversed the suppressive effects of LPS, FlaB, and CpG on development of α-GalCer-induced AHR. The reduced numbers of α-GalCer-induced inflammatory cells caused by intranasal LPS, FlaB, or CpG treatment returned to those of α-GalCer-induced inflammatory cells in mice treated with IL-12 mAb, but not the IgG2b isotype. The reduced levels of IL-4, IL-13, and IFN-γ caused by intranasal LPS, FlaB, or CpG treatment returned to those of α-GalCer-induced cytokines in mice treated with IL-12 mAb, but not the IgG2b isotype (Figs. 4-6). These data indicate that intranasal LPS, FlaB, or CpG treatment may suppress the function of iNKT cells in the airways in an IL-12-dependent manner.
We examined whether the IL-12-dependent suppressive effect of intranasal treatment with LPS, FlaB, or CpG on airway iNKT cells was also reproduced in spleen iNKT cells. Intracellular cytokines of iNKT cells were stained in α-GalCer-stimulated spleen cell cultures pretreated with LPS, FlaB, or CpG in the presence and absence of IL-12 mAb (Fig. 7A). Numbers of IL-4+ or IFN-γ + iNKT cells were increased in α-GalCer-stimulated cells compared with non-stimulated cells, but decreased in α-GalCer-stimulated cells that had been pretreated 24 hours earlier with LPS, compared with α-GalCer-stimulated cells. However, the addition of anti-IL-12 mAb, but not isotype, to LPS-pretreated and α-GalCer-stimulated spleen cells reversed the reduced number of IL-4+ or IFN-γ + cells to the level in α-GalCer-stimulated spleen cells (Fig. 7B and C). These findings indicate that LPS treatment suppresses the function of spleen iNKT cells in an IL-12-dependent manner.
Pretreatment with FlaB decreased the number of IL-4+ or IFN-γ + iNKT cells in α-GalCer-stimulated spleen cells. The reduced number of IL-4+ or IFN-γ + cells was restored to the level in α-GalCer-stimulated spleen cells by addition of IL-12 mAb, but not the IgG2b isotype (Fig. 7D and E). These data suggest that FlaB treatment suppresses the function of spleen iNKT cells in an IL-12-dependent manner.
Pretreatment with CpG decreased the number of IL-4+ or IFN-γ + iNKT cells in α-GalCer-stimulated spleen cells. The reduced number of IL-4+ or IFN-γ + cells was restored to the level in α-GalCer-stimulated spleen cells by addition of anti-IL-12 mAb, but not isotype (Fig. 7F and G). These findings indicate that CpG treatment may suppress the function of spleen iNKT cells in an IL-12-dependent manner.
In the present study, treatment with a TLR4, 5, or 9 agonist inhibited the function of iNKT cells in both airways and spleen, which was abrogated by treatment with IL-12 mAb, suggesting that the TLR agonists inhibited the function of iNKT cells in an IL-12-dependent fashion in asthma. Thus, these three TLR agonists may be used as an immunomodulator for iNKT cells in asthma.
All three TLR agonists increased production of IL-12 in BAL fluid, which was consistent with the findings of other studies.18,25,26 We wondered how IL-12 could inhibit production of both IL-4 and IFN-γ from α-GalCer-stimulated iNKT cells. The finding that IL12 mAb treatment eliminated the suppressive effect of IL-12 on production of IL-4 and IFN-γ may suggest a requirement for IL-12 in the suppressed production of Th1 and Th2 cytokines. This phenomenon may be explained by iNKT cell anergy or hyporesponsiveness induced by IL-12. Anergy is one of the mechanisms of peripheral T cell tolerance. Exposure of CD4+ T cells to an antigen may make the cells incapable of subsequently responding to that antigen.27 Some studies have suggested that stimulation of iNKT cells with α-GalCer may result in an anergic state to subsequent stimulation following the initial activation.21-23 iNKT cell anergy has been associated with down-regulation of invariant T cell receptor.21 The kinetics of initial iNKT cell activation, expansion, and induction of anergy in response to α-GalCer stimulation has been suggested to resemble the responses of T cells to superantigen.22 iNKT cells might be activated by microorganisms non-specifically via production of proinflammatory cytokines by dendritic cells (DCs), such as type I IFN28 or IL-12.18,19,29 A recent study has demonstrated that mouse iNKT cells activated by LPS or flagellin become anergic to subsequent activation with α-GalCer, and that IL-12 may play a critical role in induction of iNKT cell anergy.19 Thus, the IL-12 produced by DCs stimulated with TLR agonists may activate iNKT cells non-specifically, resulting in induction of iNKT cell anergy. It has been reported that IL-12-induced iNKT cell anergy may be associated with the downregulation of NK1.1, the activating NK cell receptor NKG2D and the CD94 subunit of the NKG2/CD94 family of NK cell receptors, may be induced independently of thymus, may be predominantly iNKT cell autonomous, and can been overcome by treatment with reagents that can bypass early T cell receptor signaling events.19 However, further studies are needed to elucidate the precise mechanisms of iNKT cell anergy.
However, IL-12 may lead to production of IFN-γ from iNKT cells. LPS-stimulated DCs produce IL-12, which induced IFN-γ, but not IL-4, production by iNKT cells.18,29 CpG-stimulated DCs also activate NKT cells via IL-12 secretion, in which NKT cells produce IFN-γ, but not IL-4.20 In contrast, in the present study, IL-12 inhibited production of both IL-4 and IFN-γ. It seems reasonable to suggest that IL-12-activated iNKT cells may produce IFN-γ at an earlier stage, but that iNKT cell anergy develops at a later stage. However, treatment with LPS, flagellin, or CpG alone did not increase IFN-γ levels in BAL fluid obtained after two days. IFN-γ production would be found to have increased had the levels been measured at an earlier time point. IFN-γ cytokine production by iNKT cells has been reported to peak 12 hours after α-GalCer stimulation.30
Various infectious stimuli, such as TLR ligands, may be expected to suppress asthma and allergic diseases. Indeed, LPS has been reported to attenuate allergic airway diseases.15 We previously reported that intranasal administration of flagellin may inhibit development of allergic asthma.16 CpG may suppress development of allergic asthma.17 Our results may in part explain why treatment with LPS, flagellin, and CpG suppresses development of allergic asthma., Indeed, a TLR7 agonist was recently reported to inhibit development of allergic asthma.31
Taken together, our data support the view that TLR4, 5, and 9 agonists may suppress the function of airway and spleen iNKT cells via IL-12-dependent mechanisms. iNKT cell anergy induced by IL-12 may play a role in the suppression by TLR agonists. Thus, LPS, flagellin, and CpG, as new immunomodulators of iNKT cells, may be used to control asthma. Further studies will be needed to elucidate how precisely IL-12 induces iNKT cell anergy.
Figures and Tables
 | Fig. 1Effect of lipopolysaccharide (LPS) on α-galactosylceramide (GalCer)-induced airway hyperreactivity (AHR), airway inflammation, and cytokine production. Naïve mice were intranasally administered α-GalCer or phosphate-buffered saline (PBS). Twenty-four hours later, AHR to methacholine (A), inflammatory cells (B), and cytokines (C, D, and E) in bronchoalveolar lavage (BAL) fluid were measured. LPS was intranasally administered 24 hr before α-GalCer administration. LPS alone denotes administration of LPS without α-GalCer. Enhanced pause (Penh) was expressed as the percentage increase in Penh, where Penh after PBS represented 100%. Total cells, macrophages (Mac), eosinophils (Eos), neutrophils (Neut), and lymphocytes (Lymph) were expressed as the number per mL BAL fluid. Data are expressed as the mean±standard error of the mean (SEM; n=5). *P<0.01 for α-GalCer vs. PBS; †P<0.05 and ‡P<0.01 for LPS + α-GalCer vs. α-GalCer; and §P<0.05 and ∥P<0.01 for LPS alone vs. α-GalCer. |
 | Fig. 2Effect of flagellin B (FlaB) on α-GalCer-induced AHR, airway inflammation, and cytokine production. Naïve mice were intranasally administered α-GalCer or PBS. Twenty-four hours later, AHR to methacholine (A), inflammatory cells (B), and cytokines (C, D, and E) in BAL fluid were measured. FlaB was intranasally administered 24 hr before α-GalCer administration. FlaB alone denotes the administration of FlaB without α-GalCer. Penh was expressed as the percentage increase in Penh, where Penh after PBS represented 100%. Total cells, Mac, Eos, Neut, and Lymph were expressed as the number per mL BAL fluid. Data are expressed as the mean±SEM (n=5). *P<0.05 and †P<0.01 for α-GalCer vs. PBS; ‡P<0.05 and §P<0.01 for FlaB + α-GalCer vs. α-GalCer; and ∥P<0.05 and ¶P<0.01 for FlaB alone vs. α-GalCer. |
 | Fig. 3Effect of CpG on α-GalCer-induced AHR, airway inflammation and cytokine production. Naïve mice were intranasally administered α-GalCer or PBS. Twenty-four hours later, AHR to methacholine (A), inflammatory cells (B), and cytokines (C, D, and E) in BAL fluid were measured. CpG was intranasally administered 24 hr before α-GalCer administration. CpG alone denotes the administration of FlaB without α-GalCer. Penh was expressed as the percentage increase in Penh, where Penh after PBS represented 100%. Total cells, Mac, Eos, Neut, and Lymph were expressed as the number per mL BAL fluid. Data are expressed as the mean±SEM (n=5). *P<0.05 and †P<0.01 for α-GalCer vs. PBS; ‡P<0.05 and §P<0.01 for CpG + α-GalCer vs. α-GalCer; and ∥P<0.05 and ¶P<0.01 for CpG alone vs. α-GalCer. |
 | Fig. 4Blocking effect of IL12 mAb on the suppressive effect of lipopolysaccharide (LPS) on α-GalCer-induced AHR, airway inflammation and cytokine production. As described in Fig. 1, AHR to methacholine (A), inflammatory cells (B) and cytokines (C, D, and E) in BAL fluid were measured. IL-12 mAb or the IgG2b isotype was intraperitoneally administered immediately before LPS administration. Penh was expressed as the percentage increase in Penh, where Penh after PBS represented 100%. Total cells, Mac, Eos, Neut, and Lymph were expressed as the number per mL BAL fluid. Data are expressed as the mean±SEM (n=5). *P<0.05 and †P<0.01 for anti-IL-12 or the isotype vs. LPS + α-GalCer. |
 | Fig. 5Blocking effect of IL12 mAb on the suppressive effect of FlaB on α-GalCer-induced AHR, airway inflammation, and cytokine production. As described in Fig. 2, AHR to methacholine (A), inflammatory cells (B) and cytokines (C, D, and E) in BAL fluid were measured. IL-12 mAb or the IgG2b isotype was intraperitoneally administered immediately before FlaB administration. Penh was expressed as the percentage increase in Penh, where Penh after PBS represented 100%. Total cells, Mac, Eos, Neut, and Lymph were expressed as the number per mL BAL fluid. Data are expressed as the mean±SEM (n=5). *P<0.05 and †P<0.01 for anti-IL-12 or the isotype vs. FlaB + α-GalCer. |
 | Fig. 6Blocking effect of IL12 mAb on the suppressive effect of CpG on α-GalCer-induced AHR, airway inflammation, and cytokine production. As described in Fig. 3, AHR to methacholine (A), inflammatory cells (B), and cytokines (C, D, and E) in BAL fluid were measured. IL-12 mAb or the IgG2b isotype was intraperitoneally administered immediately before CpG administration. Penh was expressed as the percentage increase in Penh, where Penh after PBS represented 100%. Total cells, Mac, Eos, Neut, and Lymph were expressed as the number per mL BAL fluid. Data are expressed as the mean±SEM (n=5). *P<0.05 and †P<0.01 for anti-IL-12 or the isotype vs. CpG + α-GalCer. |
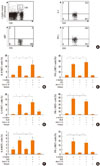 | Fig. 7Effects of LPS, FlaB, or CpG on IL-4 and IFN-γ production from α-GalCer-stimulated spleen invariant natural killer T (iNKT) cells and the blocking effect of IL12 mAb. Spleen cells were stimulated with α-GalCer and, 90 min later, intracellular cytokines of iNKT cells were stained. LPS, FlaB, or CpG was added 16 hr before α-GalCer stimulation. IL-12 or the IgG2b isotype was added immediately before addition of LPS, FlaB, or CpG. CD3+ α-GalCer-loaded CD1d tetramer+ iNKT cells were gated and the proportion of IL-4+ or IFN-γ + iNKT cells was analyzed, in which IgG1 isotypes were used for IL-4 and IFN-γ mAbs (A). IL-4+ (B) and IFN-γ + (C) iNKT cells were measured in cultures treated with LPS. IL-4+ (D) and IFN-γ + (E) iNKT cells were measured in cultures treated with FlaB. IL-4+ (F) and IFN-γ + (G) iNKT cells were measured in cultures treated with CpG. Spleen cells were pooled from three mice, and data are expressed as the mean±SEM of triplicate measurements. *P<0.05. |
ACKNOWLEDGMENTS
This study was supported financially by the Special Research Program of Chonnam National University (2009) and in part by the Regional Technology Innovation Program of the Ministry of Knowledge Economy of the Republic of Korea (No. RTI 05-01-01).
References
1. Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002. 2:557–568.
2. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003. 9:582–588.
3. Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006. 103:2782–2787.
4. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008. 205:385–393.
5. Koh YI, Kim HY, Meyer EH, Pichavant M, Akbari O, Yasumi T, Savage PB, Dekruyff RH, Umetsu DT. Activation of nonclassical CD1d-restricted NK T cells induces airway hyperreactivity in beta 2-microglobulin-deficient mice. J Immunol. 2008. 181:4560–4569.
6. Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlström J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006. 354:1117–1129.
7. Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedmann PS, Djukanovic R. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007. 356:1410–1422.
8. Koh YI, Shim JU, Wi JO, Han ER, Jin NC, Oh SH, Park CK, Park DJ. Inverse association of peripheral blood CD4(+) invariant natural killer T cells with atopy in human asthma. Hum Immunol. 2010. 71:186–191.
9. Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010. 153:239–248.
10. Koh YI, Shim JU, Wi J, Kwon YE. The Role of Natural Killer T Cells in the Pathogenesis of Acute Exacerbation of Human Asthma. Int Arch Allergy Immunol. 2012. 158:131–141.
11. Matsuda H, Suda T, Sato J, Nagata T, Koide Y, Chida K, Nakamura H. alpha-Galactosylceramide, a ligand of natural killer T cells, inhibits allergic airway inflammation. Am J Respir Cell Mol Biol. 2005. 33:22–31.
12. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006. 355:2226–2235.
13. Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, Redegeld FA. Mechanisms of allergy and asthma. Eur J Pharmacol. 2008. 585:354–360.
14. Matricardi PM, Bjorksten B, Bonini S, Bousquet J, Djukanovic R, Dreborg S, Gereda J, Malling HJ, Popov T, Raz E, Renz H, Wold A. EAACI Task Force 7. Microbial products in allergy prevention and therapy. Allergy. 2003. 58:461–471.
15. Bortolatto J, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, Mucida D, Gomes E, Christ A, Schnyder-Candrian S, Schnyder B, Ryffel B, Russo M. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clin Exp Allergy. 2008. 38:1668–1679.
16. Lee SE, Koh YI, Kim MK, Kim YR, Kim SY, Nam JH, Choi YD, Bae SJ, Ko YJ, Ryu HJ, Koh JT, Choy HE, Rhee JH. Inhibition of airway allergic disease by co-administration of flagellin with allergen. J Clin Immunol. 2008. 28:157–165.
17. Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998. 161:7054–7062.
18. Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007. 178:2706–2713.
19. Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008. 118:2301–2315.
20. Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008. 181:4452–4456.
21. Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004. 16:241–247.
22. Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005. 115:2572–2583.
23. Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005. 175:3092–3101.
24. Koh YI, Shim JU, Lee JH, Chung IJ, Min JJ, Rhee JH, Lee HC, Chung DH, Wi JO. Natural killer T cells are dispensable in the development of allergen-induced airway hyperresponsiveness, inflammation and remodelling in a mouse model of chronic asthma. Clin Exp Immunol. 2010. 161:159–170.
25. Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galán JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006. 103:12487–12492.
26. Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009. 182:1846–1853.
27. Schwartz RH. T cell anergy. Annu Rev Immunol. 2003. 21:305–334.
28. Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007. 27:597–609.
29. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003. 4:1230–1237.
30. Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS, Kang CY. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol. 2008. 180:2062–2068.
31. Grela F, Aumeunier A, Bardel E, Van LP, Bourgeois E, Vanoirbeek J, Leite-de-Moraes M, Schneider E, Dy M, Herbelin A, Thieblemont N. The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-gamma. J Immunol. 2011. 186:284–290.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download