Abstract
Purpose
The pathogenesis of nasal polyposis (NP) is unclear. Eosinophils and mast cells are considered to play important roles in this process. In addition, the levels of Th2-type cells are increased, irrespective of the atopic status of the patient with NP. In this context, we and others have shown high levels of thymus and activation-related chemokine/CCL17, macrophage-derived chemokine, eotaxin, and RANTES in patients with NP. Forkhead box P3 (FOXP3) plays a key role in CD4+CD25+ regulatory T-cell function and represents a specific marker for regulatory T cells (Tregs). Decreased expression of FOXP3 has been reported in allergic diseases. The present study was designed to evaluate the presence and potential roles of Tregs, defined by the expression of FOXP3 protein, in NP.
Methods
Using immunohistochemistry, we estimated the numbers of FOXP3+ cells in the epithelium and lamina propria of the NPs of 17 patients with chronic rhinosinusitis with NP and the nasal mucosa of 15 patients with allergic rhinitis (AR). The number of FOXP3+ cells in NPs was compared with that in the nasal mucosa of patients with AR, and the numbers of FOXP3+ cells in atopic and non-atopic NP were also compared.
Results
The number of FOXP3+ cells in the lamina propria of patients with NP was significantly lower than that in the nasal mucosa of the AR patients (2.79 vs. 5.99, P=0.008). There was no statistically significant difference noted for the numbers of FOXP3+ cells between the epithelium of the NP and the nasal mucosa (3.60 vs. 2.39, P=0.180). Furthermore, the numbers of CD4+FOXP3+ cells were lower in NPs than in the allergic nasal mucosa. There was no difference in the number of FOXP3+ cells between the atopic and non-atopic NP patients.
Conclusions
Fewer Tregs (i.e., decreased FOXP3 expression) are found in NPs than in the nasal mucosa of AR patients. As the severity of eosinophilic, Th2-type inflammation and the levels of inflammatory mediators are much higher in NPs than in the nasal mucosa of AR patients, an inverse co-relationship may exist between these parameters and the number of Tregs. The deficiency of Tregs in NP may account for the more pronounced Th2-type inflammation seen in these patients.
Nasal polyposis (NP), which is a heterogeneous, chronic, inflammatory disease of the sinuses, is often associated with asthma1,2 and is characterized by a mixed Th1 and Th2 immune response.2,3 Although the precise mechanisms underlying the pathologic mechanisms of NP are not well-defined, nasal polyps have recently been reported to be characterized by the presence of numerous eosinophils, activated mast cells, and Th2-type cells.2-4 In addition, the numbers of Th2-type cells are increased, irrespective of the patient's atopic status. Studies have also demonstrated strong local upregulation of IL-13, thymus and activation-related chemokine (TARC), and immunoglobulin E (IgE) synthesis with the formation of specific IgE to Staphylococcus aureus enterotoxins, suggesting a possible role for superantigens in these pathologic processes.5,6 Recent studies have also suggested that S. aureus secretes exotoxins that act as superantigens and upregulate the variable beta region of lymphocytes in chronic hyperplastic sinusitis with nasal polyposis.7
More recently, we have demonstrated increased expression of thymic stromal lymphopoietin (TSLP) in nasal polyps, irrespective of the atopic status of the patient, as compared to the level of expression in allergic nasal mucosa.8 The level of TSLP expression was in good correlation with the levels of eosinophils and IgE in the nasal polyps, suggesting a crucial role for TSLP in driving the severity of Th2-type inflammation in the nasal polyps and increased eosinophilia.
Forkhead box P3 (FOXP3) plays important roles in the development and function of CD4(+) regulatory T cells (Tregs) and represents a specific marker for these cells. Tregs are important in balancing immune responses and maintaining peripheral tolerance. Defects in Tregs have been implicated in the pathogenesis of chronic inflammatory and autoimmune diseases, such as idiopathic thrombocytopenic purpura, burning mouth syndrome, and allograft rejection, which have decreased expression of FOXP3.9-11 This may reflect the importance of FOXP3 for the maintenance of normal tissues. Current evidence suggests that allergic disease and asthma are also characterized by deficiencies in Tregs, which allow Th2 cells to expand.12-14 Despite the observed increases inTh2-type cells and IgE levels in the nasal polyps, irrespective of the patient's atopic status, there are no studies comparing the numbers of Tregs in nasal polyps and allergic nasal mucosa.
To study the role of FOXP3 in the pathogenesis of nasal polyps, we compared the levels of FOXP3 expression in the nasal polyps and nasal mucosa of patients with allergic rhinitis.
Seventeen patients with NP (12 males and 5 females; mean age, 30.6 years) were included in this study. Two of the enrolled patients had asthma. Seven of the patients with NP were atopic with perennial allergic rhinitis (PAR) and ten of them were non-atopic. Six of the PAR patients with NP had typical nasal allergy to house dust mite (HDM) and one had nasal allergy to cat and dog dander, as diagnosed using history and the radioallergosorbent test (RAST). Of the six patients with HDM allergy, four also had seasonal allergic rhinitis to Japanese cedar pollen. In the present study, we also included fifteen patients with PAR (13 males and 2 females; mean age, 28.75 years) who had typical clinical symptoms of nasal allergy, comprising sneezing, rhinorrhea and nasal congestion, and positive serum-specific IgE, as analyzed by RAST.
The diagnosis of allergic rhinitis (AR) was based on a history of clinical symptoms of sneezing, rhinorrhea, and nasal congestion, clinical examination by anterior rhinoscopy and, when there was a positive history, a positive serum-specific IgE by RAST. All patients were symptomatic at the time of collecting the specimens and none of them had previously received immunotherapy. Nasal polyp specimens and nasal mucosal tissue samples were collected at surgery performed as part of the treatment either for the removal of nasal polyps or for the resection of hypertrophied turbinates. All medications were prohibited for at least one month prior to surgery. The study was approved by the Nippon Medical School Medical Ethics Committee and informed consent was obtained from all patients.
Nasal polyp tissues were obtained at surgery done as a part of the treatment for the removal of nasal polyps (polypectomy/functional endoscopic sinus surgery). Nasal mucosal specimens were obtained at surgery (conchotomy) performed for the treatment of hypertrophied turbinates. Both nasal polyp specimens and nasal mucosal tissue specimens (3×4 mm) were rinsed in phosphate-buffered saline (PBS; pH 7.6), and then processed for immunohistochemistry as described below.
The nasal polyp and nasal mucosa tissue specimens were first fixed in periodate lysine paraformaldehyde (PLP), and then incubated for 4 hours each in gradients of sucrose that varied from 10% to 20% in PBS. Subsequently, the specimens were embedded in Tissue Tech OCT compound, frozen in liquid nitrogen, and stored at -80℃ until further use. To investigate the expression of FOXP3 and to localize the protein in the nasal polyps and nasal mucosa, immunohistochemistry was performed.
For immunohistochemistry, frozen sections of PLP-fixed specimens were cut at 5-µm thickness using a cryostat (Sakura Finetek, Tokyo, Japan), air-dried for 30 minutes, and then fixed in acetone for 10 minutes. Immunohistochemistry was performed using the peroxidase-based avidin-biotin complex (ABC) method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, USA). Briefly, acetone-fixed sections were rehydrated by incubation in Tris-buffered saline (TBS; pH 7.4). Thereafter, the specimens were incubated in 10% H2O2 for 30 minutes to block endogeneous peroxidase. After two rinses in TBS, the specimens were incubated for 45 minutes in Protein Block Serum Free Ready to Use (Dako, Glostrup, Denmark). After two rinses in TBS, the sections were incubated overnight at 4℃ in the optimal concentration of primary mouse monoclonal antibody against FOXP3 (5 µg/mL) (clone 236A/E7, sc-56680, lot # K2707; Santa Cruz Biotechnology, Santa Cruz, CA, USA). IHC staining was then performed using the HRP method according to the manufacturer's instructions. After rinsing in TBS that contained 0.1% Tween-20 (TBS-t; pH 7.6), the sections were incubated in the ABC. The sections were then rinsed in TBS-t. Specific immunoreactivity of the anti-FOXP3 antibodies was visualized by incubating the tissues in multiple-adsorption biotinylated secondary horse anti-mouse IgG antibody (1:200 dilution, Vectastain Elite ABC Reagent; Vector Laboratories) and the 3-amino-9-ethyl carbazole (AEC) substrate chromogen (Ready to Use kit; Dako). All rinses were carried out in TBS-t (pH 7.6) at room temperature. The reaction was stopped by immersing in distilled water. The sections were counterstained with Mayer's hematoxylin and mounted in glycergel mounting medium (C0563; Dako). As a negative control, the primary antibodies were substituted with an irrelevant isotype-matched mouse IgG (IgG1; Dako).
Although FOXP3 is a biomarker of Tregs, it also expressed in some non-CD4+ cells.16 To confirm that the FOXP3+ cells are Tregs, we assessed FOXP3 and CD4 expression by double-immunohistochemistry using the labeled streptavidin biotin (LSAB) and alkaline phosphatase methods, so as to identify FOXP3+ CD4+ cells.15 Processing of the tissue samples for double-immunohistochemistry was performed as previously described for single staining for FOXP3. For double-immunohistochemistry, we performed the first immunostaining with HRP, as in the previous single immunohistochemistry, using HRP label detection with 3,3'-diaminobenzidine (DAB-brown) for the first primary FOXP3 mouse mAb (clone 236/E7; AbD Serotec, Kidlington, UK). Subsequently, the tissue sections were incubated in an elution buffer of 10 mM citrate and 0.05% Tween-20 (pH6.0), which had been heated to 60℃ for 10 minutes before the second immunohistochemistry, which involved the alkaline phosphatase-labeled detection system with Fast Red Substrate Systems (Dako) for the anti-CD4 mouse mAb (clone MT 310, sc-19641; Santa Cruz Biotechnology). For the negative control, the second primary antibodies were substituted with an irrelevant isotype-matched mouse IgG (IgG1) (Dako).
The numbers of positively stained FOXP3+ cells and FoxP3+ CD4+ cells were counted under an Olympus microscope in six randomly selected visual fields using an objective micrometer in an area of 0.202 mm2 and at a magnification of 400 HPF. The average for the six fields was taken as the cell count for that section.
Epithelia and lamina propria were evaluated separately. The epithelium, defined as the area between the cilia and basal membrane, was evaluated by counting positive cells per 0.0625 mm2. The lamina propria, defined as the area immediately underneath the basal membrane, was evaluated by counting positively stained cells in six randomly selected areas per high-power field (area: one high-power field at 400× is equivalent to 0.0625 mm2). The results are expressed as the number of cells per 0.0625 mm2 in the epithelial and subepithelial areas and compared between the nasal mucosa and nasal polyp tissues.
The data are presented as mean±SD. Statistical analyses were carried out using the Student's t-test. Differences were considered statistically significant only when the P-values were less than 0.05. All statistical analyses were performed using the SPSS ver. 15 software (SPSS, Chicago, IL, USA).
A large proportion of the CD4+ cells expressed FOXP3 (Fig. 3A and B). Almost all the FOXP3+ cells were CD4+. The negative control (isotype-matched IgGs) did not show immunoreactivity for either FOXP3 or CD4 (Fig. 3C).
The number of FOXP3+ cells in the epithelia of the nasal polyps group was 2.39±2.33 cells per 0.0625 mm2. In the lamina propria, the number of FOXP3+ cells was 2.79±1.74 per 0.0625 mm2 (Fig. 4A). No statistically significant difference was detected for the number of FOXP3+ cells between the epithelia and lamina propria.
The number of FOXP3+ cells in the epithelia of the nasal mucosa of allergic patients was 3.60±2.64 cells per 0.0625 mm2. The number of FOXP3+ cells in the lamina propria of the nasal mucosa of allergic patients (5.99±4.29 cells) was significantly higher than that in the epithelial layer (P=0.009) (Fig. 4B).
The numbers of FOXP3+ cells were significantly lower in the lamina propria of the nasal polyps than in the lamina propria of the nasal mucosa of the AR patients (2.79±1.74 vs. 5.99±4.29, P=0.008) (Fig. 5A). Although the number of FOXP3+ cells was higher in the epithelia of the allergic nasal mucosa, as compared to that in the nasal polyps (3.60±2.64 vs. 2.39±2.33), the difference was not statistically significant (Fig. 5B).
There was no difference in the numbers of FOXP3+ cells in the nasal polyps obtained from atopics and non-atopics, with respect to both the epithelia (2.70±1.90 vs. 2.07±2.71) and lamina propria (3.52±2.04 vs. 2.25±1.41).
Double immunohistochemistry for FOXP3 and CD4 was performed to detect cells that stained positively for both FOXP3 and CD4. The number of FOXP3+CD4+ cells was significantly higher in the nasal mucosa of the AR patients than in the nasal polyps (84.83%±5.73% vs. 63.18%±15.59%, P=0.002) (Fig. 6).
Tregs represent a subset of CD4+ cells that suppresses the functions of other lymphocytes and are characterized by surface expression of CD4 and CD25 and nuclear expression of the transcription factor FOXP3. Recently, many functions of FOXP3 and its influence on the immune system have been elucidated. Tregs influence the development and expression of atopy and the allergic response.9-11 Adaptive FOXP3+ Tregs are essential for establishing mucosal tolerance and for suppressing IL-4 production.17 Decreased expression of FOXP3 has been reported in asthma and allergic rhinitis,12,18 although there have been no studies to compare FOXP3 expression in nasal polyps and allergic nasal mucosa or to define the role of Tregs in NP pathogenesis. In the present study, we compared the levels of FOXP3 expression in allergic nasal mucosa and nasal polyps and demonstrated lower expression of FOXP3 in nasal polyps.
Recently, NPs were characterized by a polarized Th2 reaction and eosinophilic inflammation and high IgE levels, irrespective of atopic status. We have recently reported that high-level expression of TSLP correlates strongly with the numbers of eosinophils in nasal polyps and also IgE, irrespective of atopic status.8 In the present study, we demonstrate decreased numbers of FOXP3+ cells in nasal polyps, as compared to allergic nasal mucosa. These lower numbers of FOXP3+ cells observed in patients with NP may result in unopposed overexpression of Th2 cells and the increased migration and activation of dendritic cells, eosinophils, and mast cells.
High IgE concentrations have been found in nasal polyps, indicating that IgE is produced locally.19-21 Suh et al.20 also reported a polyclonal hyper-immunoglobulinemia E, associated with the presence of IgE specific for S. aureus enterotoxins (SAE), colonization with S. aureus, and increased eosinophilic inflammation in a relevant subgroup (about 50%) of nasal polyp patients. These authors also reported a strong correlation between the levels of IgE in nasal polyps and the presence of nasal eosinophils. Our recent study demonstrates that the high levels of IgE seen in nasal polyps are in good correlation with the number of TSLP+ cells in nasal polyps as well as between TSLP and eosinophils, irrespective of atopic status.8 Moreover, the levels of TARC/CCL17 and macrophage-derived chemokine (MDC) were also high in the nasal polyps.8 In the present study, we also found lower numbers of CD4+FOXP3+ cells in the nasal polyps than in the allergic nasal mucosa, irrespective of atopic status.
It has been suggested that S. aureus secretes exotoxins that may act as superantigens and upregulate the variable beta region of lymphocytes in chronic hyperplastic sinusitis with NP.7 Recently, Van Bruaene et al.22 reported lower expression of FOXP3 mRNA in chronic rhinosinusitis. In the present study, we compared the levels of expression of FOXP3 in the nasal mucosa of patients with AR and NP, as well as in the nasal polyps from atopics and non-atopics. While the numbers of Tregs in the nasal mucosa of patients with AR are reported to be low, we detected a decrease in the number of CD4+FOXP3+ Tregs in both the atopic and non-atopic nasal polyps. In this context, Pérez Novo et al.23 recently assessed the effect of S. aureus enterotoxin B (SEB) on T-cell activation in patients with nasal polyps and asthma, and its possible link to aspirin hypersensitivity. SEB significantly increased the levels of IFN-γ, IL-4, TNF-α, IL-5, and IL-2 in the supernatants of both NP polyp groups with asthma but with and without aspirin sensitivity compared with controls. The baseline level of FOXP3 was significantly decreased in both NP-asthma groups. After incubation with SEB, FOXP3 was significantly up-regulated in the control group, but not in the NP-asthma groups. These researchers concluded that although SEB induces both Th1 and Th2 pro-inflammatory responses in patients with NP and asthma, regardless of the presence of aspirin hypersensitivity, the nature of this response may be linked to the basal deficiency of FOXP3 observed in the NP-asthma patients. Therefore, the deficiency of FOXP3 may lead to the enhanced Th2 inflammation in the NP of atopics and non-atopics and the high level of IgE, irrespective of the atopic status.
These data suggest that the suppressive function of Tregs may be partially inhibited. This might partially explain the more pronounced Th2-type inflammation and eosinophilic inflammation in nasal polyps, as compared to the allergic nasal mucosa. Furthermore, there is no reported statistically significant difference between the lymphocyte subpopulations of atopic and non-atopic patients.24,25
Taken together, the present findings indicate that the deficiency of Tregs in nasal polyps may play an important role in enhancement of the severity of Th2 inflammation in nasal polyps and the persistence and progression of NP. Furthermore, these results point to increasingly important roles for T cells in chronic rhinosinusitis with nasal polyposis.
Figures and Tables
 | Fig. 1Immunoreactivity and localization of forkhead box P3 (FOXP3) protein in nasal polyps. The immunoreactivity for FOXP3 was analyzed by immunohistochemistry using the peroxidase-based avidin-biotin complex method, as described in the text. FOXP3 immunoreactivity is shown in (A), with FOXP3 expression being detected mainly in the immune cells of the lamina propria. (B) The negative control shows no immunoreactivity for FOXP3. Magnification ×400 HPF. |
 | Fig. 2Immunoreactivity and localization of forkhead box P3 (FOXP3) in the nasal mucosa of patients with allergic rhinitis. Immunoreactivity for FOXP3 was analyzed by immunohistochemistry using the peroxidase-based avidin-biotin complex method, as described in the text. FOXP3 immunoreactivity is shown in (A), with many FOXP3-expressing cells being evident in the lamina propria. (B) The negative control shows no immunoreactivity for FOXP3. Magnification ×400 HPF. |
 | Fig. 3Immunoreactivity and localization of forkhead box P3 (FOXP3)+CD4+ cells. Double-staining using labeled streptavidin biotin (LSAB) detection with 3,3'-diaminobenzidine (DAB-brown) for FOXP3 expression and LSAB alkaline phosphatase detection with Fast Red substrate (red) for CD4+ cells was performed as described in the text (A). Symbols used: ☆, FOXP3+/CD4+ cell; △, FOXP3-/CD4+ cell. (B) Double-staining with anti-FOXP3 mAb and IgG1 (negative control for CD4). The arrow indicates FOXP3+ cells. Immunoreactivity is evident for only FOXP3+ cells. There is no immunoreactivity for IgG1. (C) The negative control for both FOXP3 and CD4 shows no immunoreactivity for FOXP3 or CD4. Magnification ×400 HPF |
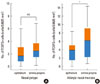 | Fig. 4(A) The numbers of forkhead box P3 (FOXP3)-positive cells in the epithelia and lamina propria of nasal polyps. Immunoreactivity for FOXP3 was analyzed by immunohistochemistry using the peroxidase-based avidin-biotin complex (ABC) method, as described in the text. Positively stained cells were counted as described in the text. Magnification ×400 HPF. There is no statistically significant difference between the numbers of FOXP3+ cells in the epithelia and lamina propria. (B) The numbers of FOXP3+ cells in the epithelia and lamina propria of the nasal mucosa of patients with allergic rhinitis. Immunoreactivity for FOXP3 was analyzed by immunohistochemistry using the peroxidase-based ABC method, as described in the text. Positively stained cells were counted as described in the text. Magnification ×400 HPF. The number of FOXP3+ cells is significantly higher in the lamina propria than in the epithelium. *P<0.01. |
 | Fig. 5(A) Comparison of the numbers of forkhead box P3 (FOXP3)-positive cells in the lamina propria of nasal polyps and allergic nasal mucosa. Immunoreactivity for FOXP3 was analyzed by immunohistochemistry using the peroxidase-based avidin-biotin complex (ABC) method, as described in the text. Positively stained cells were counted as described in the text. Magnification ×400 HPF. The number of FOXP3+ cells is significantly lower in the lamina propria of the nasal polyps than in those of the allergic nasal mucosa. *P<0.01. (B) Comparison of the numbers of FOXP3+ cells in the epithelia of nasal polyps and allergic nasal mucosa. Immunoreactivity for FOXP3 was analyzed by immunohistochemistry using the peroxidase-based ABC method, as described in the text. Positively stained cells were counted as described in the text. Magnification ×400 HPF. Although the number of FOXP3+ cells is higher in the epithelia of the allergic nasal mucosa than in those of the nasal polyps, the difference is not statistically significant (NS). |
 | Fig. 6Comparison of the numbers of forkhead box P3 (FOXP3)-positive cells in the epithelia of nasal polyps and allergic nasal mucosa. Double-staining for FOXP3 and CD4 was performed as described in the text. The number of FOXP3+CD4+ cells in the nasal mucosa of allergic rhinitis patients is significantly higher than that in the nasal polyps. *P<0.01. |
ACKNOWLEDGMENTS
This study was supported in part by the Society for Promotion of International Otorhinolaryngology (SPIO). The authors thank Sachiko Saito, Miyuki Takatori, and Naoko Minematsu for technical assistance with the laboratory study.
References
1. Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, Schleimer RT, Kern RC. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009. 23:145–148.
2. Pawankar R, Nonaka M. Inflammatory mechanisms and remodeling in chronic rhinosinusitis and nasal polyps. Curr Allergy Asthma Rep. 2007. 7:202–208.
3. Sánchez-Segura A, Brieva JA, Rodríguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol. 1998. 102:953–960.
4. Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP). Clin Exp Allergy. 1998. 28:1145–1152.
5. Patou J, Gevaert P, Van Zele T, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008. 121:110–115.
6. Fukumoto A, Nonaka M, Ogihara N, Pawankar R. Induction of TARC production by lipopolysaccharide and interleukin-4 in nasal fibroblasts. Int Arch Allergy Immunol. 2008. 145:291–297.
7. Bernstein JM, Allen C, Rich G, Dryja D, Bina P, Reiser R, Ballow M, Wilding GE. Further observations on the role of Staphylococcus aureus exotoxins and IgE in the pathogenesis of nasal polyposis. Laryngoscope. 2011. 121:647–655.
8. Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, Okubo K. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011. 3:186–193.
9. Ling Y, Cao XS, Yu ZQ, Luo GH, Bai X, Su J, Dai L, Ruan CG. Alterations of CD4+ CD25+ regulatory T cells in patients with idiopathic thrombocytopenic purpura. Zhonghua Xue Ye Xue Za Zhi. 2007. 28:184–188.
10. Pekiner FN, Demirel GY, Gümrü B, Ozbayrak S. Serum cytokine and T regulatory cell levels in patients with burning mouth syndrome. J Oral Pathol Med. 2008. 37:528–534.
11. Wang J, Zhao R, Zhang F, Li J, Huo B, Cao Y, Dou K. Control of allograft rejection in mice by applying a novel neuropeptide, cortistatin. Adv Ther. 2008. 25:1331–1341.
12. Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009. 64:1539–1546.
13. Paik Y, Dahl M, Fang D, Calhoun K. Update: the role of FoxP3 in allergic disease. Curr Opin Otolaryngol Head Neck Surg. 2008. 16:275–279.
14. Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009. 10:689–695.
15. Dutsch-Wicherek M, Tomaszewska R, Lazar A, Strek P, Wicherek L, Kijowski J, Majka M. The presence of B7-H4+ macrophages and CD25+CD4+ and FOXP3+ regulatory T cells in the microenvironment of nasal polyps - a preliminary report. Folia Histochem Cytobiol. 2010. 48:611–617.
16. Mason DY, Sammons R. Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol. 1978. 31:454–460.
17. Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008. 29:114–126.
18. Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007. 148:53–63.
19. Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, van Cauwenberge P, Bachert C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004. 114:981–983.
20. Suh KS, Park HS, Nahm DH, Kim YK, Lee YM, Park K. Role of IgG, IgA, and IgE antibodies in nasal polyp tissue: their relationships with eosinophilic infiltration and degranulation. J Korean Med Sci. 2002. 17:375–380.
21. Mandron M, Ariès MF, Brehm RD, Tranter HS, Acharya KR, Charveron M, Davrinche C. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol. 2006. 117:1141–1147.
22. Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, Gevaert P. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008. 121:1435–1441. 1441.e1–1441.e3.
23. Pérez Novo CA, Jedrzejczak-Czechowicz M, Lewandowska-Polak A, Claeys C, Holtappels G, Van Cauwenberge P, Kowalski ML, Bachert C. T cell inflammatory response, Foxp3 and TNFRS18-L regulation of peripheral blood mononuclear cells from patients with nasal polyps-asthma after staphylococcal superantigen stimulation. Clin Exp Allergy. 2010. 40:1323–1332.
24. Bernstein JM, Ballow M, Rich G, Allen C, Swanson M, Dmochowski J. Lymphocyte subpopulations and cytokines in nasal polyps: is there a local immune system in the nasal polyp? Otolaryngol Head Neck Surg. 2004. 130:526–535.
25. Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003. 3:1–6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download