Abstract
Purpose
Allergic rhinitis (AR) and asthma share many characteristics, but structural changes are observed far less often in AR. Matrix metalloproteinases (MMPs) constitute a family of Zn-dependent endopeptidases that can decompose the extracellular matrix and basement membrane, and regulate cell infiltration. We analyzed the expression of MMPs and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), in allergic nasal mucosa after nasal allergen challenge (NAC) and determined their relationship to inflammatory cells.
Methods
Nasal mucosa specimens were obtained at surgery performed for hypertrophied turbinates. We performed NAC with house dust mite (HDM) allergen disks and control disks, and took biopsies at 30 minutes, 6 hours, and 12 hours after NAC. Cells expressing MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2, as well as eosinophils and mast cells, were analyzed immunohistochemically. The MMPs and TIMPs in allergic nasal mucosa were quantified using enzyme-linked immunosorbent assays.
Results
At 30 minutes post-NAC, HDM-exposed nasal mucosa exhibited significantly more MMP-2+, MMP-9+, MMP-13+, TIMP-1+, and TIMP-2+ cells compared with control mucosa, and the numbers of MMP-9+ and TIMP-1+ cells correlated strongly with the number of mast cells. At 6 hours post-NAC, the numbers of MMP+ and TIMP+ cells did not differ significantly between HDM-exposed mucosa and control mucosa, but the ratios of MMP+ cells to TIMP+ cells were higher in HDM-exposed mucosa. At 12 hours post-NAC, the number of MMP-13+ cells tended to be higher in HDM-exposed mucosa and was strongly correlated with the number of eosinophils. Quantitatively, the levels of MMP-2 and MMP-13 were significantly higher than the MMP-9 level, and the TIMP-2 level was significantly higher than the TIMP-1 level in allergic nasal mucosa.
Conclusions
We demonstrated increased expression of MMP-2, MMP-9, and MMP-13 in allergic nasal mucosa, high MMPs-to-TIMP-1 ratios, and a strong correlation between MMP-9 and mast cells and between MMP-13 and eosinophils. The imbalance between MMPs and TIMPs may contribute to the migration of inflammatory cells such as eosinophils and mast cells to the nasal mucosa of AR patients, suggesting a possible active role of MMPs in AR.
Allergic rhinitis (AR) is an inflammatory disease of the nasal mucosa caused by an allergen-IgE interaction in sensitized individuals; it is characterized by the clinical symptoms of sneezing, itching, congestion, rhinorrhea, and nasal blockage.1,2 Asthma is characterized by chronic inflammation of the lower airways and shares several characteristics with AR.3,4 In both conditions, an inflammatory response is triggered by similar factors such as allergens, leading to the increased production and release of inflammatory mediators, including interleukin (IL)-4, IL-5, IL-13, granulocyte-macrophage colony-stimulating factor, histamine, leukotrienes, prostaglandins, eotaxin, and thymus and activation-regulated chemokine, as well as the upregulation of adhesion molecules common to both asthma and AR. As a result, inflammatory cells such as eosinophils, T-cells, basophils, and mast cells begin to infiltrate both the nasal mucosa and the lungs.5,6 The recruitment and migration of inflammatory cells involve traversing capillary vessel walls and the interstitium, and require degradation of extracellular matrix (ECM) proteins by secreted matrix metalloproteinases (MMPs),7 although the precise underlying mechanism is not fully understood.
The basement membrane is composed of various substances such as cell adhesive molecules and ECM-like type IV collagen, type VII collagen, laminin, fibronectin, and heparin sulfate.8 MMPs comprise a family of Zn-dependent endopeptidases that can decompose the ECM and basement membrane.9 They participate in tissue remodeling, cell infiltration, and tumor spread. At least 23 MMP family members have been characterized.10,11 In particular, MMP-2 and MMP-9 degrade type IV and V collagens as well as elastin and thus may facilitate cell migration. In addition, MMP-2, MMP-9, and MMP-13 are thought to play key roles in tissue remodeling and repair through degradation of type IV collagen, which is the major component of the basement membrane.12
The activation of MMPs is inhibited by tissue inhibitors of metalloproteinases (TIMPs), which form a 1:1 complex with MMPs.9,10 Four different TIMPs have been identified. TIMP-1 binds to both the active and precursor forms of MMP-9, whereas TIMP-2 and TIMP-4 bind to pro-MMP-2, MMP-2, and MMP-9, which are linked to gelatinolytic activity and chronic obstructive pulmonary disease.9 Another study suggested that MMP-2 and MMP-9 are inactivated by TIMP-1 and TIMP-2.13 Loss of the coordinated expression of MMPs and TIMPs is believed to generate tissue degradation under inflammatory conditions.
Epithelial cells and fibroblasts express and release MMPs.14 Additionally, eosinophils are a major source of MMPs; MMP-9 was shown to be overexpressed by eosinophils accumulating in airway walls of asthmatics.15 Several in vitro studies have demonstrated that MMP-2 and MMP-9 are produced and activated by mast cells, and the possible involvement of mast cells in connective tissue degradation and fibrosis was suggested.16,17
In asthma, inflammation and repair of the airways are ongoing processes, involving epithelial shedding and thickening of the basement membrane.18,19 In a study that evaluated bronchial biopsies, increased levels of fibronectin and type I and type III collagen were detected in the basement membrane of asthmatic subjects compared with subjects with seasonal allergic rhinitis and healthy controls.20 However, epithelial shedding, basement membrane thickening, and fibrosis are observed far less often in AR.12,21 These differences may reflect structural differences and remodeling alterations in asthma versus AR. By contrast, nasal polyps share more features with asthma. As in asthma, nasal polyps exhibit increased MMP-9 expression and low TIMP levels.22
We hypothesized that MMPs may play a role in regulating cell infiltration in allergic nasal mucosa. To investigate this, we analyzed the expression of MMPs and TIMPs in allergic nasal mucosa after nasal allergen challenge (NAC) and determined their relationship to inflammatory cells.
Thirty patients (mean age, 26 years; range, 10-65 years) with perennial allergic rhinitis (PAR) to house dust mite (HDM) allergen were included in the present study. Of the 30 patients, 24 were males and 6 were females (male:female=4:1). Ten patients had co-morbid asthma, three had chronic rhinosinusitis, and three had atopic dermatitis. AR was diagnosed based on a typical symptom history of sneezing, rhinorrhea, and nasal congestion; clinical examination by anterior rhinoscopy; positive serum-specific IgE on a radioallergosorbent test (RAST); and positive allergy skin test results. Nineteen of the 30 subjects had seasonal AR to Japanese cedar pollen; eight, to timothy grass pollen; and seven, to ragweed pollen. All patients were symptomatic at the time nasal mucosal tissue samples were collected at surgery performed as partial treatment for hypertrophied turbinates. Another six patients (4 males and 2 females) with PAR to HDM allergen were included for the enzyme-linked immunosorbent assay (ELISA) study. Medications were prohibited for at least 1 month prior to surgery. The study was approved by the Nippon Medical School Medical Ethics Committee, and informed consent was obtained from all patients.
Nasal mucosal specimens from subjects who had severe nasal obstruction and hypertrophied turbinates due to AR were obtained at surgery (conchotomy) performed for the treatment of hypertrophied turbinates. The NAC was conducted by placing a HDM allergen disk on the anterior end of the inferior turbinate of one side and a control disk on the contralateral side at 30 minutes (n=10), 6 hours (n=10), or 12 hours (n=10) before the specimens were collected at surgery. These time points reflect the early-phase and late-phase allergic reaction. Nasal mucosa specimens were rinsed in phosphate-buffered saline (PBS, pH 7.6) and processed for immunohistochemistry as described below.
Nasal mucosa tissue specimens were fixed in periodate-lysine-paraformaldehyde (PLP) and then incubated for 4 hours each in 10%, 15%, and 20% sucrose in PBS. The specimens were embedded in Tissue-Tek OCT compound (Miles Laboratories, Inc., Elkhart, IN, USA), frozen in liquid nitrogen, and stored at -80℃ until further use.
Immunohistochemistry was performed to analyze the expression of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 in nasal mucosa challenged with allergen or control disks. Frozen sections of PLP-fixed specimens were cut at 4 µm thickness using a cryostat (Sakura Finetek, Tokyo, Japan), mounted on silane-coated slides, air-dried for 30 minutes, and then fixed in 100% acetone for 10 minutes. Immunohistochemistry for the detection of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 was performed using the alkaline phosphatase/anti-alkaline phosphatase (APAAP) method with an APAAP kit (Nichirei Bioscience, Tokyo, Japan). Briefly, acetone-fixed sections were rehydrated by incubation in Tris-buffered saline (TBS, pH 7.4), followed by incubation in 10% H2O2 for 30 minutes to block endogenous peroxidase activity. After two rinses in TBS, the sections were incubated in Protein Block Serum (Dako Japan, Tokyo, Japan). After two more rinses in TBS, the sections were incubated for 12 hours at 4℃ with a primary antibody: mouse anti-human MMP-2, mouse anti-human MMP-9, mouse anti-human MMP-13, mouse anti-human TIMP-1, or mouse anti-human TIMP-2 antibody (Daiichi Fine Chemical, Toyama, Japan) at the optimal concentration (10 µg/mL). Then, the sections were rinsed twice in TBS, incubated in rabbit anti-mouse immunoglobulin (Nichirei Bioscience, Tokyo, Japan) for 30 minutes at room temperature, rinsed twice in TBS, and incubated in APAAP regent (Nichirei Bioscience) for 30 minutes at room temperature, followed by incubation with 3-amino-9-ethylcarbaxol (AEC) substrate (Dako Japan). After being rinsed twice in distilled water (DW), the sections were incubated for 30 seconds in Mayer's hematoxylin, rinsed twice in DW, and mounted in Dako gel (Dako). For the negative control, the primary antibodies were substituted with an irrelevant isotype-matched mouse IgG (IgG1; Dako).
Immunohistochemistry to detect tryptase expression (mast cells) was performed using the same method as described above, using mouse anti-human mast cell tryptase (Chemicon International, Temecula, CA, USA) at the optimal concentration (10 µg/mL) as the primary antibody. For the negative control, the primary antibody was substituted with an irrelevant isotype-matched mouse IgG (IgG1; Dako).
To investigate the expression and localization of MBP in nasal mucosa, immunohistochemistry was performed using the peroxidase-based avidin-biotin complex (ABC) method (ABC kit; Vector Laboratories, Burlingame, CA, USA). Briefly, the specimens were incubated in 10% H2O2 for 30 minutes to block endogenous peroxidase activity, rinsed twice in TBS, and incubated in Protein Block Serum (Dako). After two rinses in TBS, the sections were incubated with the primary antibody, mouse anti-human MBP (BD Biosciences, CA, USA), at an optimal concentration (5 µg/mL) for 12 hours at 4℃. The sections were rinsed twice in TBS and incubated with biotin-labeled horse anti-mouse immunoglobulin (Dako) for 30 minutes at room temperature. After two rinses in TBS, the sections were incubated in ABC reagent (Dako) for 30 minutes at room temperature, followed by incubation with the ABC substrate. Finally, the sections were rinsed twice in DW, counterstained with Mayer's hematoxylin, and mounted in Dako gel (Dako). For the negative control, the primary antibody was substituted with an irrelevant isotype-matched mouse IgG (IgG1; Dako).
The number of cells staining positive for MMP-2, MMP-9, MMP-13, TIMP-1, TIMP-2, tryptase, or MBP in each of 10 randomly selected visual fields (0.202 mm2 each) of each section was counted under an Olympus microscope (Olympus, Tokyo, Japan) at ×400 high power field (HPF) magnification using an objective micrometer. The number of positively stained cells in a section is expressed as the mean±standard error (SE) of the 10 cell counts for that section.
Unchallenged nasal mucosa tissues from six additional patients with PAR to HDM allergen were homogenized using ultrasonification. The samples were clarified by high-speed centrifugation, and the supernatants were collected and stored at -20℃ until further use. The levels of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 in nasal mucosa extracts were measured using specific ELISA kits according to the manufacturer's instructions (Amersham Biosciences, Piscataway, NJ, USA). Each sample was analyzed in duplicate. The total protein in the nasal mucosa was analyzed using a BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The levels of MMPs and TIMPs are expressed as ng/mg protein.
Statistical significance for intergroup comparisons was determined by the Wilcoxon signed-rank test. Correlation was determined using Spearman's coefficient test. Differences were deemed to be significant for values of P<0.05. All statistical analyses were performed using statistical program software (OMS, Saitama, Japan).
MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 were all expressed in the nasal mucosa of PAR patients (Fig. 1). Morphologically, all five proteins were observed mainly in inflammatory cells. In addition, MMP-2 was also present in epithelial cells, fibroblasts, and endothelial cells; MMP-9, in epithelial cells, fibroblasts, and acinar cells; MMP-13, in fibroblasts, endothelial cells, and acinar cells; and TIMP-1 and TIMP-2, in fibroblasts, epithelial cells, and endothelial cells. The negative control showed no immunoreactivity for MMP-2, MMP-9, MMP-13, TIMP-1, or TIMP-2.
The numbers of cells staining positive for MMPs and TIMPs in the allergic nasal mucosa of PAR patients at 30 minutes post-NAC are shown in Fig. 2A and 2B. Compared with the nasal mucosa exposed to the control disk, the nasal mucosa exposed to the HDM allergen disk at 30 minutes exhibited significantly more cells expressing MMP-2 (12.9±3.36 vs. 5.3±1.69, HDM vs. control; P<0.02), MMP-9 (9.8±2.52 vs. 5.1±1.41, HDM vs. control; P<0.05), MMP-13 (14.1±3.37 vs. 8.3±1.55, HDM vs. control; P<0.05), TIMP-1 (13.6±3.4 vs. 4.5±1.26, HDM vs. control; P<0.02), and TIMP-2 (15.2±3.68 vs. 5.0±1.24, HDM vs. control; P<0.02).
The numbers of cells staining positive for MMPs and TIMPs in the allergic nasal mucosa of PAR patients at 6 hours post-NAC are shown in Fig. 3A and 3B. After 6 hours, there was no significant difference between the HDM-exposed and control-exposed nasal mucosa with respect to the number of cells expressing MMP-2 (16.8±2.88 vs. 11.7±2.42, HDM vs. control), MMP-9 (7.8±3.6 vs. 5.3±1.8, HDM vs. control), MMP-13 (12.9±3.43 vs. 11.7±6.16, HDM vs. control), TIMP-1 (8.6±2.88 vs. 11.6±2.99, HDM vs. control), or TIMP-2 (14.8±3.58 vs. 1.6±2.99, HDM vs. control).
The numbers of cells staining positive for MMPs and TIMPs in the allergic nasal mucosa of PAR patients at 12 hours post-NAC are shown in Fig. 4A and 4B. Although the numbers of cells expressing each of the MMPs and TIMPs at 12 hours were greater in the HDM-exposed nasal mucosa compared with the control-exposed nasal mucosa, the difference was not significant for MMP-2 (9.6±3.94 vs. 4.8±1.02, HDM vs. control), MMP-9 (8.9±3.56 vs. 3.7±1.16, HDM vs. control), MMP-13 (11.1±3.48 vs. 5.5±1.47, HDM vs. control), TIMP-1 (8.7±3.26 vs. 6.6±1.41, HDM vs. control), or TIMP-2 (9.8±2.68 vs. 8.8±1.98, HDM vs. control).
We analyzed the ratios of cells expressing MMPs to cells expressing TIMPs in the nasal mucosa of PAR patients. The MMP-2/TIMP-1, MMP-9/TIMP-1, and MMP-13/TIMP-1 ratios in the HDM-exposed mucosa were higher than the respective ratios in the control-exposed mucosa at 6 hours after NAC, but the ratios did not differ significantly (Table). However, the MMP-2/TIMP-1 and MMP-13/TIMP-1 ratios showed a tendency of difference (P=0.07).
Mast cells are increased specifically in the nasal epithelium of patients with allergic rhinitis. To understand the relationship between MMPs and the migration of mast cells to the nasal mucosa, particularly intraepithelial migration of mast cells, we analyzed the correlation between the number of cells expressing MMPs and the number of cells expressing tryptase, a marker for mast cells. At 30 minutes after NAC, the number of mast cells was strongly correlated with the number of MMP-9+ cells (P<0.05, r=0.77) and the number of TIMP-1+ cells (P<0.05, r=0.78) (Fig. 5).
To examine the relationship between MMPs and the migration of eosinophils into allergic nasal mucosa, we analyzed the correlation between the number of cells expressing MMPs and the number expressing MBP, a marker for eosinophils. At 12 hours post-NAC, the number of eosinophils and the number of MMP-13+ cells were strongly correlated (P<0.05, r=0.75) (Fig. 6).
The amounts of MMPs and TIMPs in the nasal mucosa of patients with PAR were determined by ELISA (Fig. 7). The levels of MMP-2 (60.6±18.2 ng/mL) and MMP-13 (81.2±20.2 ng/mL) were higher than the level of MMP-9 (34.6±6.8 ng/mL) (P<0.05 and P<0.02, respectively). The level of TIMP-2 (24.8±10.2 ng/mL) was higher than the level of TIMP-1 (5.6±1.2 ng/mL) (P<0.02).
MMPs represent a family of Zn-dependent endopeptidases capable of degrading essentially all components of the ECM.9 As the gelatinase subgroup of MMPs, MMP-2 and MMP-9 are also called 72-kDa gelatinase (gelatinase A) and 92-kDa gelatinase (gelatinase B), respectively. MMP-2 degrades gelatin; type IV, V, VII, X, and XI collagens; fibronectin; elastin; and laminin.10 MMP-9 degrades gelatin; type IV, V, VII, and X collagens; elastin; proteoglycan; entacin; fibronectin; and laminin.9,10 Also called collagenase-3, MMP-13 degrades various substrates, including fibrillar-type I, II, and III collagens; type IV, IX, X, and XIV collagens; gelatin; tenascin-C; fibronectin; and proteoglycan core proteins.10 Thus, all three MMPs can degrade type IV collagen, which is the major component of the basement membrane zone.
The recruitment and migration of circulating inflammatory cells (e.g., eosinophils and basophils) to sites of inflammatory reactions in AR involve traversing capillary walls and the interstitium,5 which require the degradation of ECM proteins by secreted MMPs such as MMP-2 and MMP-9, which specifically degrade native type IV and V collagens and elastin.6 Thickening of the basement membrane of the nasal mucosa and sloughed-off epithelium with areas of epithelial metaplasia are also observed in AR.2,4 These pathological changes, which are caused by MMPs secreted by epithelial cells, fibroblasts, and inflammatory cells, are examples of tissue remodeling.9,10 MMPs are also responsible for increased microvascular permeability, leading to edema and cell migration as well as ECM remodeling at the site of inflammation.8 This suggests that reducing MMP expression at sites of allergic inflammation may be an important strategy for treating symptoms of AR. In the present study, we analyzed the expression of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 in cells of the nasal mucosa of PAR patients at 30 minutes, 6 hours, and 12 hours after NAC with HDM allergens. We also analyzed their relationship to infiltrating mast cells and eosinophils in the allergic nasal mucosa.
AR is an inflammatory condition of the nasal mucosa caused by an allergen-IgE interaction in sensitized individuals. Histogical observations have revealed structural abnormalities of the nasal mucosa, including thickening of the basement membrane and structural fibrosis, which are associated with intense infiltration by several types of inflammatory cells such as eosinophils and mast cells/basophils.11,23,24 These cellular events involve extensive alteration of the tissue ECM,14 which is involved in tissue homeostasis as well as pathological conditions. Two groups of proteins, MMPs and TIMPs, are important factors for maintaining ECM homeostasis.9,25,26 MMPs are produced by numerous cell types, including fibroblasts and macrophages, in response to inflammatory stimulation, and they mediate transmigration of eosinophils and macrophages through the basement membrane to propagate inflammation.25,27
In the present study, the expression of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 was significantly increased in the nasal mucosa of PAR patients at 30 minutes after NAC, suggesting that MMP-2, MMP-9, and MMP-13 may be crucial participants in the early-phase reaction, possibly by degrading the basement membrane to facilitate inflammatory cell migration into the allergic nasal epithelial layer. There was a strong correlation between the numbers of MMP-9+ cells and mast cells at 30 minutes post-NAC. At 12 hours post-NAC, MMP-13 expression tended to be increased, and there was a strong correlation between the number of MMP-13+ cells and the number of eosinophils. This suggests that MMP-9 may have an important role in the early-phase migration of mast cells into the epithelial compartment, while MMP-13 may participate in eosinophil recruitment/migration during the late-phase reaction in allergic nasal mucosa.
High MMP-9 expression has been reported in the lower airway walls of asthmatics.7,27 In our previous study, nasal polyps exhibited much higher MMP-9 expression and lower TIMP expression compared with the present levels in nasal mucosa.22 Although the MMP-9-to-TIMP ratio was high after NAC in the present study, it was still lower than that in nasal polyps (data not shown). The expression levels of MMP-9, MMP-2, and TIMP-1 in nasal polyps of patients with comorbid AR were more prominent than those in the control group.28 More recently, increased expression of MMP-9 and TIMP-1 was detected in the nasal septal mucosa of an allergic rhinitis murine model.29 Thus, MMP-9 in allergic nasal mucosa may contribute to the migration or recruitment of eosinophils, but may not be sufficient to induce structural changes such as those observed in nasal polyps or asthma. At 6 hours post-NAC in the present study, although the numbers of MMP-2+, MMP-9+, and MMP-13+ cells were not significantly higher in HDM-exposed nasal mucosa compared with control nasal mucosa, the ratios of MMP+ cells to TIMP-1+ cells were higher in HDM-exposed mucosa. This suggests that a relatively low TIMP-1 level may facilitate the activities of MMP-2, MMP-9, and MMP-13 related to cell migration during the late-phase allergic reaction.
An imbalance between MMP-9 and TIMP-1 in induced sputum is associated with airway inflammation in asthma and chronic obstructive pulmonary disease.30 In the present study, we demonstrated for the first time that the levels of MMP-2, MMP-9 and MMP-13 are high relative to the levels of TIMP-1 and TIMP-2 in ongoing chronic inflammation of PAR and that MMP+ cells are correlated with mast cells in the early phase and with eosinophils in the late phase of the inflammatory reaction. The imbalance between MMPs and TIMPs may contribute to inflammatory cell migration in the nasal mucosa of AR patients. These findings suggest a possible active role of MMPs in the pathomechanism of AR.
Figures and Tables
 | Fig. 1Immunoreactivity and localization of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 in the nasal mucosa of PAR patients. Immunohistochemistry using the APAAP method was performed as described in the Materials and Methods section. (A) MMP-2 was expressed mainly in inflammatory cells, but also in fibroblasts, epithelial cells, and endothelial cells. (B) MMP-9 was expressed mainly in inflammatory cells, but also in fibroblasts, epithelial cells, and acinar cells. (C) MMP-13 was expressed mainly in inflammatory cells, but also in fibroblasts, endothelial cells, and acinar cells. (D) TIMP-1 was expressed mainly in inflammatory cells, but also in fibroblasts, epithelial cells, and endothelial cells. (E) TIMP-2 was expressed mainly in inflammatory cells, but also in fibroblasts and endothelial cells. (F) The negative control showed no immunoreactivity (magnification, ×200-400 HPF). |
 | Fig. 2The numbers of MMP-2+, MMP-9+, MMP-13+, TIMP-1+, and TIMP-2+ cells in the nasal mucosa of PAR patients at 30 min after NAC with HDM allergen disks versus control disks. Immunohistochemistry using the APAAP method was performed as described in the Materials and Methods section. Positively stained cells in an area of 0.202 mm2 were counted at ×400 HPF. Box plots represent the median values with 25 and 75% interquartiles. Error bars represent the 10th and 90th percentiles; the squares indicate the means. (A) At 30 min post-NAC, the numbers of MMP-2+, MMP-9+, and MMP-13+ cells in the HDM-exposed mucosa were significantly higher than those in the control-exposed mucosa (n=10). (B) At 30 min post-NAC, the numbers of TIMP-1+ and TIMP-2+ cells in the HDM-exposed mucosa were significantly higher than those in the control-exposed mucosa (n=10). *P<0.05, **P<0.02, by Wilcoxon signed-rank test. |
 | Fig. 3The numbers of MMP-2+, MMP-9+, MMP-13+, TIMP-1+, and TIMP-2+ cells in the nasal mucosa of PAR patients at 6 hr after NAC with HDM allergen disks versus control disks. Immunohistochemistry using the APAAP method was performed as described in the Materials and Methods section. Positively stained cells in an area of 0.202 mm2 were counted at ×400 HPF. Box plots represent the median values with 25 and 75% interquartiles. Error bars represent the 10th and 90th percentiles; the squares indicate the means. (A) At 6 hr post-NAC, there was no significant difference in the number of MMP-2+, MMP-9+, or MMP-13+ cells between HDM-exposed and control-exposed nasal mucosa (n=10). (B) At 6 hr post-NAC, there was no significant difference in the number of TIMP-1+ or TIMP-2+ cells between HDM-exposed and control-exposed nasal mucosa (n=10). |
 | Fig. 4The numbers of MMP-2+, MMP-9+, MMP-13+, TIMP-1+, and TIMP-2+ cells in the nasal mucosa of PAR patients at 12 hr after NAC with HDM allergen disks versus control disks. Immunohistochemistry using the APAAP method was performed as described in the Materials and Methods section. Positively stained cells in an area of 0.202 mm2 were counted at ×400 HPF. Box plots represent the median values with 25 and 75% interquartiles. Error bars represent the 10th and 90th percentiles; the squares indicate the means. (A) At 12 hr post-NAC, there was no difference in the number of MMP-2+, MMP-9+, or MMP-13+ cells between HDM-exposed and control-exposed nasal mucosa (n=10). (B) At 12 hr post-NAC, there was no difference in the number of TIMP-1+ or TIMP-2+ cells between HDM-exposed and control-exposed nasal mucosa (n=10). |
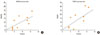 | Fig. 5Correlation of MMP-9+ and TIMP-1+ cells with mast cells in the nasal mucosa of PAR patients. (A) There was a strong correlation between the number of mast cells and the number of MMP-9+ cells at 30 min after NAC with HDM allergen disks. (r=0.8, P<0.02, by Spearman's rank correlation coefficient; n=10.) (B) There was a strong correlation between the number of mast cells and the number of TIMP-1+ cells at 30 min after NAC with HDM allergen disks. (r=0.8, P<0.05, by Spearman's rank correlation coefficient; n=10.) |
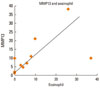 | Fig. 6Correlation between MMP-13+ cells and eosinophils in the nasal mucosa of PAR patients. The number of eosinophils was strongly correlated with the number of MMP-13+ cells at 12 hr after NAC with HDM allergen disks (r=0.75, P<0.05, by Spearman's rank correlation coefficient; n=10). |
 | Fig. 7The levels of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2, as determined by specific ELISA, in the nasal mucosa of PAR patients. MMP-2 and MMP-13 levels were higher than the MMP-9 level, and the TIMP-2 level was higher than the TIMP-1 level in allergic nasal mucosa. *P<0.05, **P<0.01, Wilcoxon signed-rank test; n=6. |
Table
Ratio of the numbers of cells expressing a MMP vs. TIMP in the nasal mucosa of PAR patients

The MMP-2/TIMP-1, MMP-9/TIMP-1, and MMP-13/TIMP-1 ratios in the HDM-exposed mucosa were higher than the respective ratios in the control-exposed mucosa at 6 hr after NAC, but theratios did not differ significantly. However, the MMP2/TIMP1 and MMP13/TIMP1 ratios showed a tendency of difference.
*P=0.07.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors express their gratitude to Ms. Sachiko Saito, Ms. Naoko Minematsu, and Ms. Miyuki Takatori for their kind technical assistance.
References
1. Howarth PH. ABC of allergies. Pathogenic mechanisms: a rational basis for treatment. BMJ. 1998. 316:758–761.
2. Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000. 55:Suppl 64. 7–16.
3. Pawankar R. Allergic rhinitis and asthma: are they manifestations of one syndrome? Clin Exp Allergy. 2006. 36:1–4.
4. Djukanović R, Wilson SJ, Howarth PH. Pathology of rhinitis and bronchial asthma. Clin Exp Allergy. 1996. 26:Suppl 3. 44–51.
5. Mygind N, Bisgaard H. Mygind N, Pipkorn U, Dahl R, editors. Applied anatomy of the airways. Rhinitis and asthma: similarities and differences. 1990. Copenhagen: Munksgaard;21–37.
6. Busse WW, Holgate ST, editors. Asthma and rhinitis. 1995. Boston: Blackwell Scientific Publications;12–624.
7. Lee KS, Jin SM, Kim HJ, Lee YC. Matrix metalloproteinase inhibitor regulates inflammatory cell migration by reducing ICAM-1 and VCAM-1 expression in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2003. 111:1278–1284.
8. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998. 102:783–788.
9. Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992. 7:120–125.
10. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997. 378:151–160.
11. Howarth PH. The cellular basis for allergic rhinitis. Allergy. 1995. 50:6–10.
12. Salib RJ, Howarth PH. Remodelling of the upper airways in allergic rhinitis: is it a feature of the disease? Clin Exp Allergy. 2003. 33:1629–1633.
13. Shimizu T, Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production in nasal fibroblasts by tranilast, an antiallergic agent, in vitro. Mediators Inflamm. 2005. 2005:150–159.
14. Hoshino M, Takahashi M, Takai Y, Sim J. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in asthma. J Allergy Clin Immunol. 1999. 104:356–363.
15. Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997. 16:212–219.
16. Kanbe N, Tanaka A, Kanbe M, Itakura A, Kurosawa M, Matsuda H. Human mast cells produce matrix metalloproteinase 9. Eur J Immunol. 1999. 29:2645–2649.
17. Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999. 162:5528–5535.
18. Vignola AM, Chiappara G, Chanez P, Merendino AM, Pace E, Spatafora M, Bousquet J, Bonsignore G. Growth factors in asthma. Monaldi Arch Chest Dis. 1997. 52:159–169.
19. Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989. 1:520–524.
20. Chakir J, Laviolette M, Boutet M, Laliberté R, Dubé J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996. 75:735–744.
21. Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004. 113:43–49.
22. Pawankar R, Watanabe S, Nonaka M, Ozu C, Aida M, Yagi T. Differential expression of matrix metalloproteinase 2 and 9 in the allergic nasal mucosa and nasal polyps. J Allergy Clin Immunol. 2004. 113:S332.
23. Calderón MA, Lozewicz S, Prior A, Jordan S, Trigg CJ, Davies RJ. Lymphocyte infiltration and thickness of the nasal mucous membrane in perennial and seasonal allergic rhinitis. J Allergy Clin Immunol. 1994. 93:635–643.
24. Pawankar R. Inflammatory mechanisms in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2007. 7:1–4.
25. Shaida A, Kenyon G, Devalia J, Davies RJ, MacDonald TT, Pender SL. Matrix metalloproteinases and their inhibitors in the nasal mucosa of patients with perennial allergic rhinitis. J Allergy Clin Immunol. 2001. 108:791–796.
26. Herouy Y, Mellios P, Bandemir E, Dichmann S, Nockowski P, Schöpf E, Norgauer J. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J Dermatol Sci. 2001. 25:198–205.
27. Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax. 1999. 54:590–596.
28. Shin HW, Han DH, Lim YS, Kim HJ, Kim DY, Lee CH, Min YG, Rhee CS. Nonasthmatic nasal polyposis patients with allergy exhibit greater epithelial MMP positivity. Otolaryngol Head Neck Surg. 2009. 141:442–447.
29. Moon IJ, Kim DY, Rhee CS, Lee CH, Min YG. Role of angiogenic factors in airway remodeling in an allergic rhinitis murine model. Allergy Asthma Immunol Res. 2012. 4:37–45.
30. Erlewyn-Lajeunesse MD, Hunt LP, Pohunek P, Dobson SJ, Kochhar P, Warner JA, Warner JO. Bronchoalveolar lavage MMP-9 and TIMP-1 in preschool wheezers and their relationship to persistent wheeze. Pediatr Res. 2008. 64:194–199.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download