Abstract
Purpose
Methods
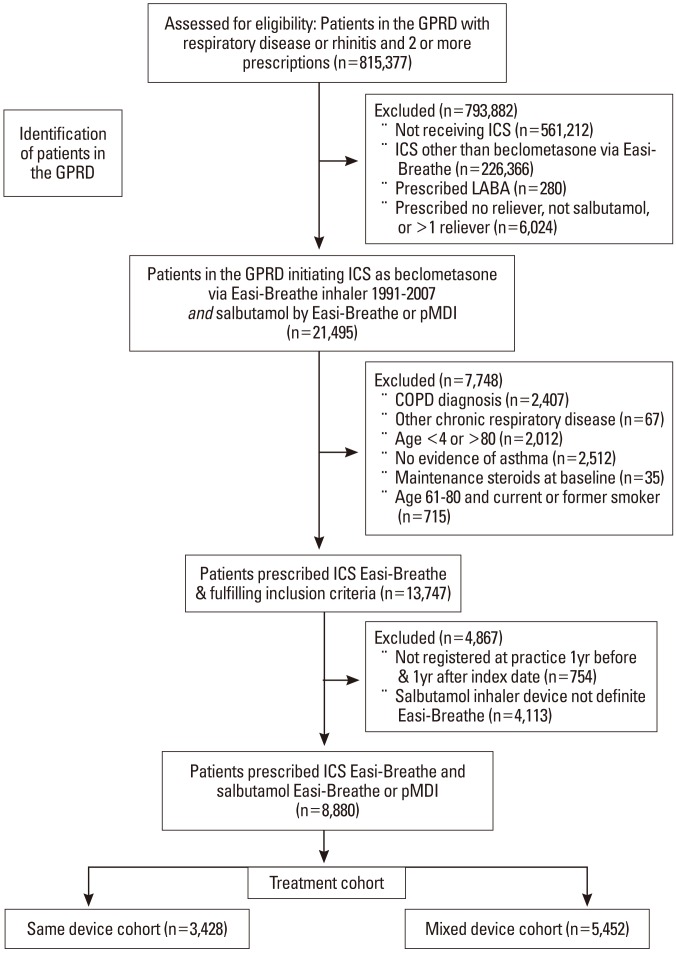
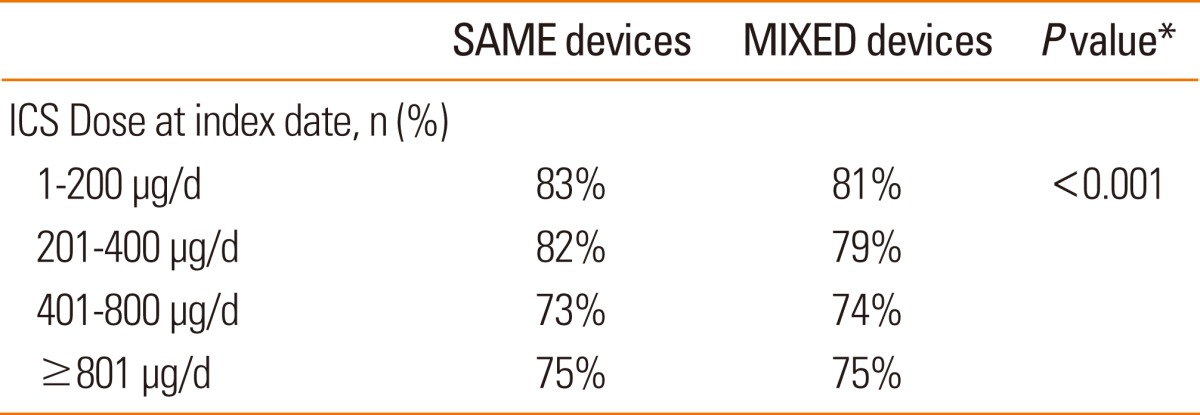
Results
ACKNOWLEDGMENTS
References
Fig. 2
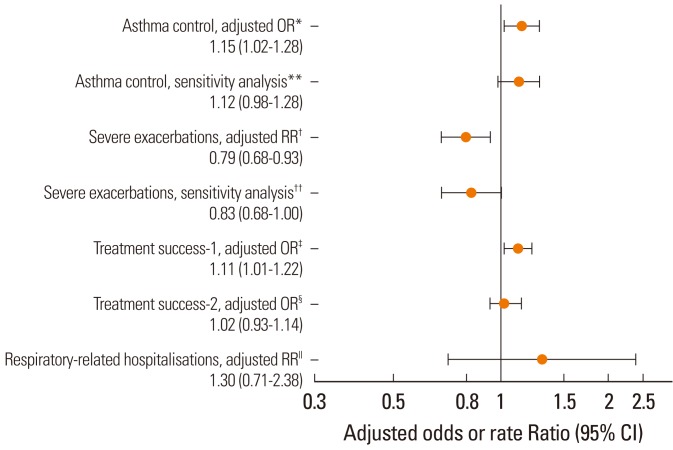
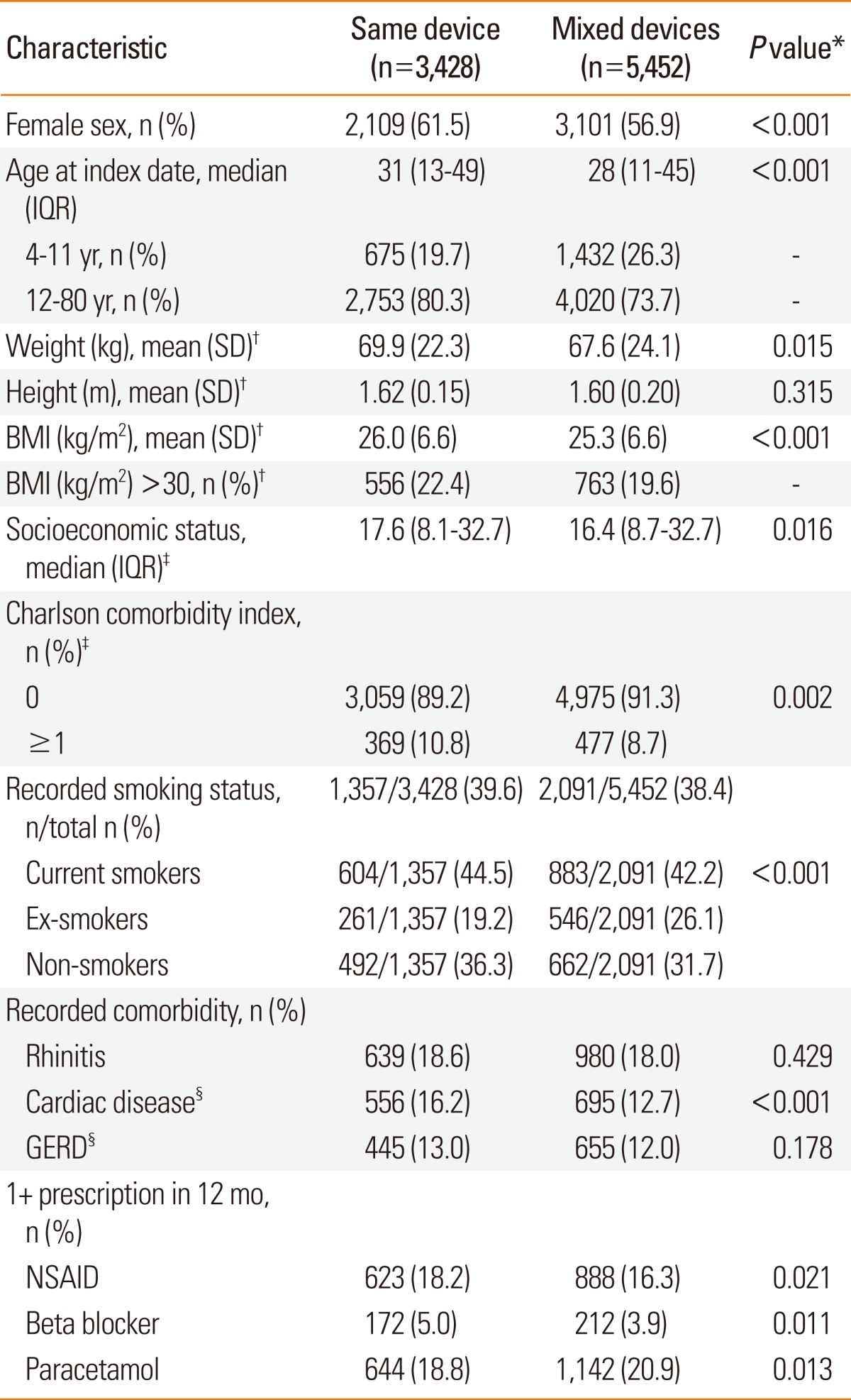
Table 1

*Co-primary endpoints; †Treatment success-2 differs from treatment success-1 in excluding the changes in therapeutic regimen that could be attributed to cost-saving measures.
A&E, Accident & Emergency; ICS, inhaled corticosteroid; LRTI, lower respiratory tract infection; LTRA, leukotriene receptor antagonist; OPD, Outpatient Department.
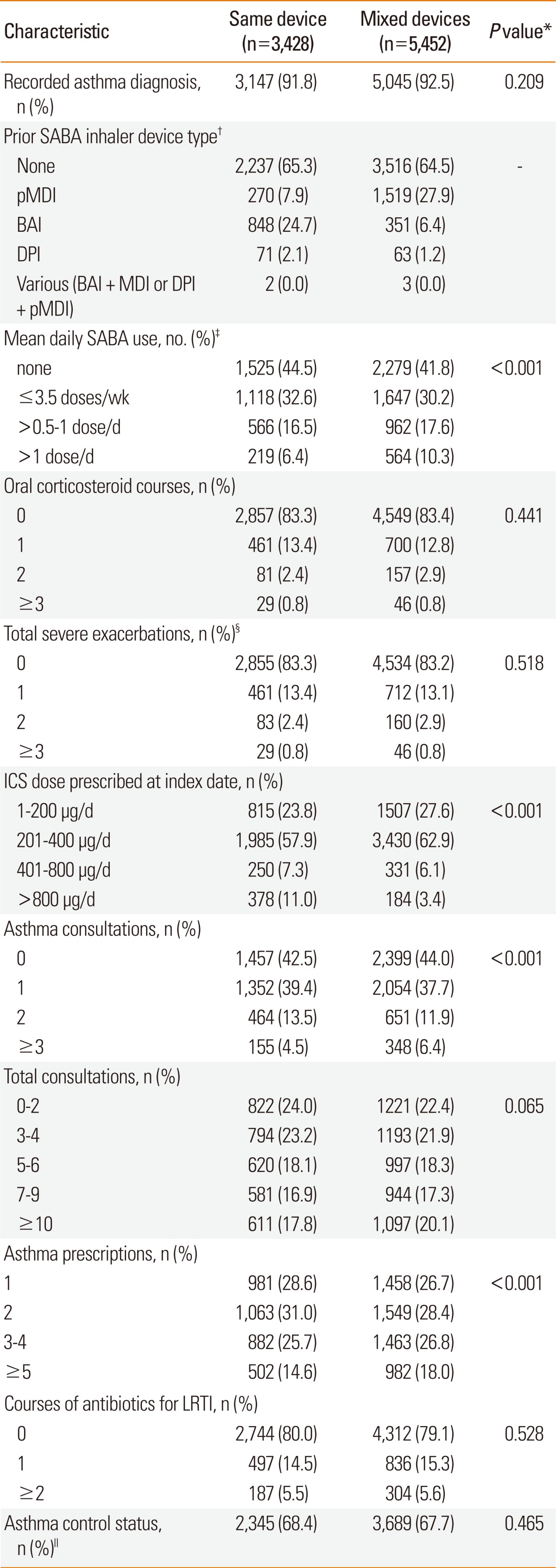
Table 2
*Categorical values were compared with the χ2 test and continuous variables with the Mann-Whitney test; †Weight and height were recorded closest to the index date; for children <12, weight and height were included only if within 2 years of index date. Not all patients had recorded weight and height data. For weight, n=2,564 (74.8%) and 3,984 (73.1%); height n=2,696 (78.6%) and 4,225 (77.5%); BMI n=2,483 (72.4%) and 3886 (71.3%) for same and mixed devices cohort, respectively; ‡Socioeconomic status was that assigned, in quintiles, by the General Practice Research Database to each practice using the Index of Multiple Deprivation as a proxy measure. The Charlson comorbidity index is a weighted index that accounts for number and severity of comorbidities, each assigned a score depending on the associated risk of dying; §Patients with cardiac disease and GERD included those with a recorded diagnosis or recorded prescription for same.
BAI, breath-actuated inhaler; BMI, body mass index; GERD, gastro-oesophageal reflux disease; ICS, inhaled corticosteroid; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; pMDI, pressurised metered-dose inhaler; SD, standard deviation.
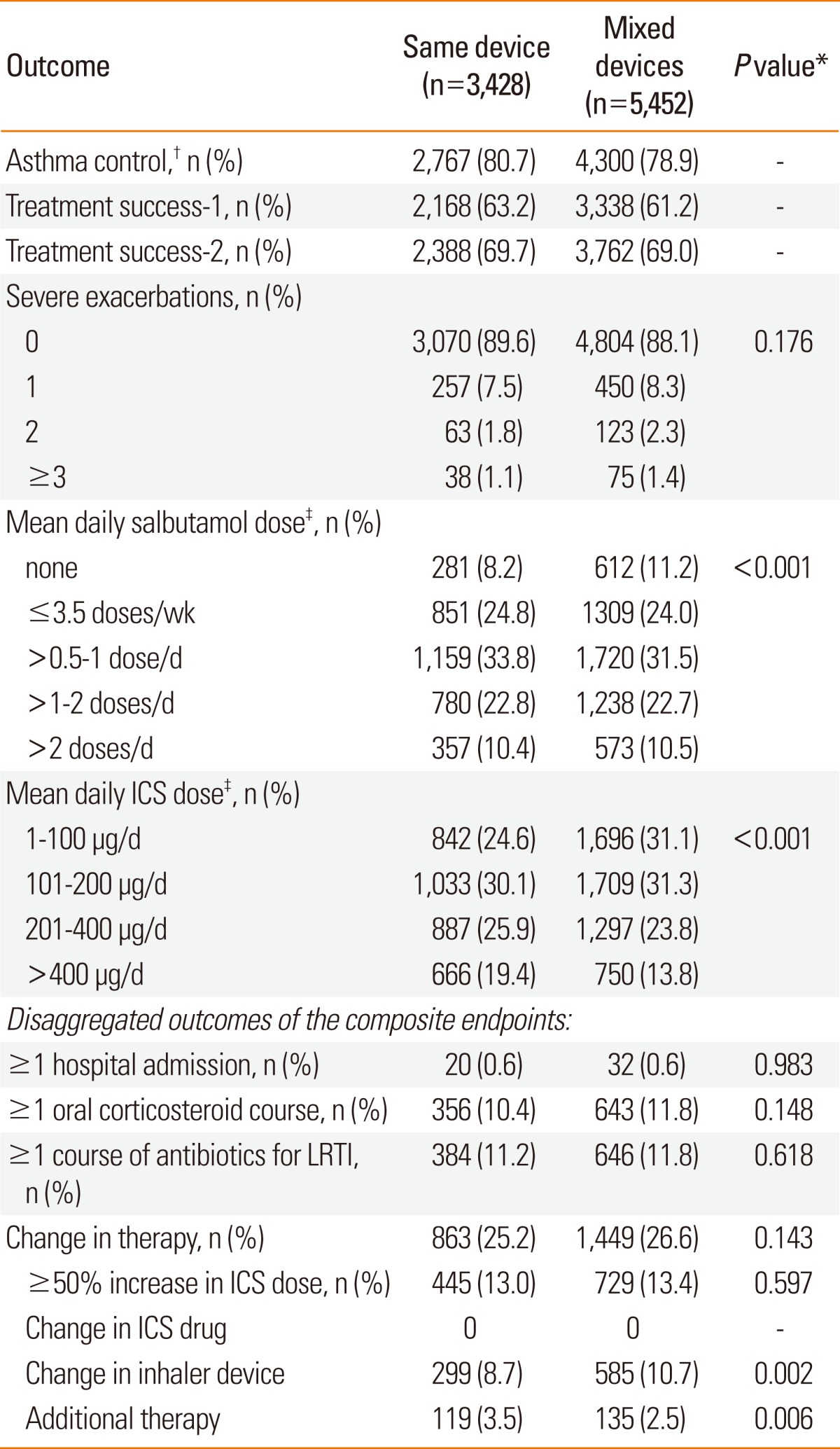
Table 3
*Categorical values were compared with the χ2 test and continuous variables with the Mann-Whitney test; †The prior SABA device type was included only for the baseline year; thus, because some patients had a pre-baseline SABA prescription, ~55% of patients used SABA during the baseline year but only ~35% of patients had a SABA prescription in the baseline period; ‡The SABA dose is the salbutamol dose equivalent (standard dose in UK is 200 µg). Thus, patients who used ≤3.5 doses/wk averaged salbutamol 1-100 µg/d; those who used >0.5-1 dose/d, 101-200 µg/d; and those who used >1 dose/d, >200 µg/d; §A severe exacerbation was defined as an occurrence of unscheduled hospital admission or emergency room attendance for asthma or prescription for oral corticosteroids; exacerbations on the index date were included in the baseline data; ∥Asthma control was defined as no hospital attendance for asthma, oral corticosteroid course, or antibiotics for LRTI during the baseline year.
BAI, breath-actuated inhaler; DPI, dry powder inhaler; ICS, inhaled corticosteroid; LRTI, lower respiratory tract infection; pMDI, pressurised metered-dose inhaler; SABA, short-acting β2-agonist.
Table 4
*Categorical values were compared with the χ2 test and continuous variables with the Mann-Whitney test; †See Table 1 for definitions of study endpoints; ‡The standard dose of salbutamol in the UK is 200 µg. Thus, patients who used ≤3.5 doses/wk averaged 1-100 µg/d; those who used >0.5-1 dose/d, 101-200 µg/d; those who used >1-2 doses/d, 201-400 µg/d; and those who used >2 doses/d, >400 µg/d. The daily salbutamol and ICS doses consumed during the outcome year were calculated as the dispensed amount divided by 365.
ICS, inhaled corticosteroid; LRTI, lower respiratory tract infection.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download