Abstract
Thromboembolism is one of the most critical complications of hypereosinophilic syndrome (HES). We report here a case of multi-organ infarctions related to HES. A 23-year-old woman was referred to our hospital with hemoptysis. Not only pulmonary, but also renal and splenic infarctions were detected on computed tomography images. Blood tests showed profound peripheral eosinophilia. She was diagnosed with HES with disseminated intravascular coagulation (DIC). We initiated infusion of corticosteroids, which effectively suppressed peripheral eosinophilia. However, consumptive coagulopathy did not improve and intracerebral hemorrhage related to thrombosis then developed. Addition of interferon-alpha resulted in the correction of the DIC associated with HES.
Idiopathic hypereosinophilic syndrome (HES) is characterized by persistent blood and tissue eosinophilia. HES involves multiple organ systems, predominantly the skin, heart, lung, gastrointestinal tract, and nervous system.1 Often, the presenting manifestation is thrombosis. Sporadic cases of intra-abdominal, cerebral, and cutaneous thrombosis have been reported.2 Disseminated intravascular coagulation (DIC) associated with HES is rare, and few cases have been reported.3-6 Here we report a unique case of HES presenting with pulmonary, renal, splenic, and intracerebral thrombosis associated with DIC.
A 23-year-old woman was referred to the emergency department of our hospital with fever and hemoptysis. She had bilateral costovertebral angle tenderness. A simple chest X-ray demonstrated patchy consolidation on the right middle lobe and peribronchial opacity on the lower bilateral lung fields (Fig. 1A). A chest computed tomography (CT) scan revealed a heavy burden of pulmonary embolism in the bilateral pulmonary arteries and their branches, with bland infarction in both lungs (Fig. 1B-D). In an abdominal-pelvic CT scan to evaluate the patient's flank pain, a focal wedge-shaped low-density lesion in the right kidney midpole and spleen was found (Fig. 2), suggesting infarction. A duplex ultrasonogram of the lower extremities for the edematous right lower leg revealed a totally occluding thrombus of the right femoropopliteal vein and a partially occluding thrombus of the right posterior tibial vein.
White blood cell count was elevated (11,430/µL) with 21% eosinophils (2,550/µL), hemoglobin 11 g/dL, and thrombocytopenia of 39,000/µL. The prothrombin time (PT) was prolonged to 15 seconds and activated partial thromboplastin time was 42 seconds. Fibrinogen was in the normal range (156 mg/dL), but D-dimer was increased to 46.14 µg/mL. Total IgE was 2,296 IU/mL and ECP was 78.6 ng/mL. Anti-neutrophil cytoplasmic and cardiolipin antibodies were negative. A test for parasites in the stool and serum was negative. A bone marrow biopsy showed normal cellularity with increased eosinophils. Fip1-like1 and platelet-derived growth factor receptor alpha gene (FIP1L1-PDGFRA) fusion was not detected. An echocardiogram showed no abnormal findings.
She was diagnosed as idiopathic HES manifested as multiple thromboembolisms involving the lung, kidney, spleen, and lower extremities. She was treated with corticosteroids (methylprednisolone 1 mg/kg) and heparin on the second day of admission. Eosinophil count normalized within 3 days. On the fifth day of treatment, she complained of a persistent and worsening headache. A brain CT scan revealed left frontal and temporal lobe intracerebral hemorrhage. D-dimer remained elevated at 18.93 µg/mL, and the platelet count was low at 30,000/µL. Due to suspicion of hemorrhage associated with intracerebral thrombosis, we continued corticosteroid and anticoagulation therapy. On the ninth day of treatment, a follow-up brain CT scan showed no interval change of hemorrhage. Although the peripheral eosinophil count was within the normal range, thrombocytopenia (48,000/µL) and D-dimer elevation (60 µg/mL) remained abnormal. We started subcutaneous injection of interferon-alpha (INF-α) (3 MU/day), after which the thrombocytopenia and elevation of D-dimer gradually normalized (Fig. 3). The patient's headache and abdominal pain were then completely absent. INF-α injections were stopped after 5 days and corticosteroids were gradually tapered off without any recurrence of symptoms or abnormal laboratory findings.
Since 1975, three criteria have been used to define HES: blood eosinophilia >1,500/µL for longer than 6 months; lack of evidence of parasitic, allergic, or other known causes of eosinophilia; and presumptive signs of organ involvement.7 Our case fulfilled the diagnostic criteria of HES with the exception of duration. However, the relative importance of HES duration is controversial. Simon et al.8 emphasized the importance of effective therapies to halt progression of organ damage that can occur with HES, rather than waiting if the criterion of duration has not been met.
The patient initially presented with pulmonary, renal, and splenic infarctions associated with eosinophilia and consumptive coagulopathy. Consumptive coagulopathy, also called DIC, is characterized by abnormal activation and consumption of clotting factors. Thromboembolism is one of the most serious complications of HES. About 25% of HES patients develop thromboembolism, and 5%-10% die as a result.2 The mechanisms
underlying the thrombotic diathesis in HES are not fully understood, but the four main granule proteins released by eosinophils-major basic protein (MBP), eosinophil derived neurotoxin (EDN), eosinophil cationic protein (ECP), and eosinophil peroxidase (EPO)-are thought to cause hypercoagulation. ECP has been reported to promote coagulation through a factor XII-dependent mechanism.9 MBP and EPO are known to activate platelets.10 MBP can inhibit the anticoagulant activities of the endothelial membrane by binding to thrombomodulin. 11,12 Moreover, hypothiocyanous acid (HOSCN), the predominant oxidant product of EPO, has been shown to stimulate tissue factor expression, thus promoting thrombosis.13
Corticosteroids are the first-line therapy for FIP1L1-PDGFRA-negative HES, and are very effective for reducing peripheral eosinophils.14 If corticosteroid-resistance is observed, second-line therapies such as INF-α, hydroxyurea, and anti-IL-5 monoclonal antibodies are warranted. In this case, peripheral eosinophil counts were normalized immediately by corticosteroid administration. However, the consumptive coagulopathy was not corrected, and intracranial hemorrhage developed, which must have been related to thrombosis. Peripheral eosinophil count does not always parallel tissue damage. Kobayashi et al.15 reported a case of idiopathic HES manifesting as acute abdominal pain caused by thrombosis of the mesenteric arteries. Although the peripheral eosinophilia was profoundly reduced by corticosteroid administration, the patient needed emergency laparotomy for the intestinal perforation. There is another report of progressive ischemia in the extremities with HES requiring amputation of the legs, despite the use of corticosteroids.16
In our case, administration of INF-α resulted in a restoration of platelet count and a decreased D-dimer level. INF-α is effective for treatment of corticosteroid-resistant HES.17-20
In summary, the case we present here shows that activated eosinophils can provoke thromboembolism in various organs in HES. If consumptive coagulopathy is not controlled by administration of corticosteroids alone, addition of an immunomodulatory therapy, such as INF-α, should be considered.
Figures and Tables
Fig. 1
Chest x-ray showed patchy consolidation on the right middle lobe and increased peribronchial opacity on lower bilateral lung fields (A). Chest CT showed large thrombus (arrows) in the right interlobar artery (B) and combined pulmonary infarction (arrowhead) (C). Another thrombus (arrows) was seen in the left interlobar artery (D).
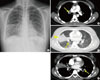
Fig. 2
Abdominal-pelvic CT scan showed focal wedge shaped low density lesion (arrows) in the right kidney midpole (A, C) and spleen (B) suggesting infarctions.
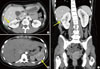
Fig. 3
Disseminated intravascular coagulation profiles according to the patient progress. Platelet count, D-dimer, and fibrinogen level were gradually recovered after addition of interferon-alpha. *Gray bar, the duration of corticosteroid treatment; yellow bar, the duration of interferon-alpha treatment; INF-¶, interferon-alpha; HD, hospital day.

ACKNOWLEDGMENTS
This study was supported by a Samsung Medical Center Clinical Research Development Program grant, #CRS1092011.
References
1. Klion A. Hypereosinophilic syndrome: current approach to diagnosis and treatment. Annu Rev Med. 2009. 60:293–306.
2. Ogbogu PU, Rosing DR, Horne MK 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007. 27:457–475.
3. Yeung TF, Lau SW, Wong K. An unusual case of hypereosinophilic syndrome and disseminated intravascular coagulation. Chin Med J (Engl). 2005. 118:1582–1584.
4. Miyagi J, Ichimiya M, Ozaki K, Goto T, Fujino O, Nagata J, Hiasa Y. Hypereosinophilic syndrome complicated by disseminated intravascular coagulation (DIC), deep venous thrombosis and pulmonary embolism. Nihon Naika Gakkai Zasshi. 2004. 93:364–366.
5. Nagashima M, Nishizawa M, Yamauchi T, Mori S, Honma Y. A case of the idiopathic hypereosinophilic syndrome presenting with mononeuritis multiplex, multiple thrombosis, and disseminated intravascular coagulation. Rinsho Shinkeigaku. 1986. 26:698–703.
6. Fukuta A, Hara T, Tsurumi H, Moriwaki H. Hypereosinophilic syndrome with DIC treated successfully with a combination of high-dose methylprednisolone and cyclosporin A. Rinsho Ketsueki. 2001. 42:1145–1147.
7. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975. 54:1–27.
8. Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, Rosenwasser LJ, Roufosse F, Gleich GJ, Klion AD. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010. 126:45–49.
9. Venge P, Dahl R, Hallgren R. Enhancement of factor XII dependent reactions by eosinophil cationic protein. Thromb Res. 1979. 14:641–649.
10. Rohrbach MS, Wheatley CL, Slifman NR, Gleich GJ. Activation of platelets by eosinophil granule proteins. J Exp Med. 1990. 172:1271–1274.
11. Slungaard A, Vercellotti GM, Tran T, Gleich GJ, Key NS. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J Clin Invest. 1993. 91:1721–1730.
12. Mukai HY, Ninomiya H, Ohtani K, Nagasawa T, Abe T. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br J Haematol. 1995. 90:892–899.
13. Wang JG, Mahmud SA, Thompson JA, Geng JG, Key NS, Slungaard A. The principal eosinophil peroxidase product, HOSCN, is a uniquely potent phagocyte oxidant inducer of endothelial cell tissue factor activity: a potential mechanism for thrombosis in eosinophilic inflammatory states. Blood. 2006. 107:558–565.
14. Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res. 2010. 2:87–101.
15. Kobayashi M, Komatsu N, Kuwayama Y, Bandobashi K, Kubota T, Uemura Y, Taguchi H. Idiopathic hypereosinophilic syndrome presenting acute abdomen. Intern Med. 2007. 46:675–678.
16. Ferguson GT, Starkebaum G. Thromboangiitis obliterans associated with idiopathic hypereosinophilia. Arch Intern Med. 1985. 145:1726–1728.
17. Ceretelli S, Capochiani E, Petrini M. Interferon-alpha in the idiopathic hypereosinophilic syndrome: consideration of five cases. Ann Hematol. 1998. 77:161–164.
18. Yamada O, Kitahara K, Imamura K, Ozasa H, Okada M, Mizoguchi H. Clinical and cytogenetic remission induced by interferon-alpha in a patient with chronic eosinophilic leukemia associated with a unique t(3;9;5) translocation. Am J Hematol. 1998. 58:137–141.
19. Fruehauf S, Fiehn C, Haas R, Doehner H, Hunstein W. Sustained remission of idiopathic hypereosinophilic syndrome following alpha-interferon therapy. Acta Haematol. 1993. 89:91–93.
20. Terrier B, Piette AM, Kerob D, Cordoliani F, Tancréde E, Hamidou L, Lebbé C, Blétry O, Kahn JE. Superficial venous thrombophlebitis as the initial manifestation of hypereosinophilic syndrome: study of the first 3 cases. Arch Dermatol. 2006. 142:1606–1610.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download