Abstract
Regional dietary habits and cooking methods affect the prevalence of specific food allergies; therefore, we determined the effects of various pH conditions on major peanut allergens. Peanut kernels were soaked overnight in commercial vinegar (pH 2.3) or acetic acid solutions at pH 1.0, 3.0, or 5.0. Protein extracts from the sera of seven patients with peanut-specific IgE levels >15 kUA/L were analyzed by SDS-PAGE and immunolabeling. A densitometer was used to quantify and compare the allergenicity of each protein. The density of Ara h 1 was reduced by treatment with pH 1.0, 3.0, or 5.0 acetic acid, or commercial vinegar. Ara h 2 remained largely unchanged after treatment with pH 5.0 acetic acid, and was decreased following treatment with pH 1.0, 2.3, or 3.0 acetic acid. Ara h 3 and Ara h 6 appeared as a thick band after treatment with pH 1.0 acetic acid and commercial vinegar. IgE-binding intensities to Ara h 1, Ara h 2, and Ara h 3 were significantly reduced after treatment with pH 1.0 acetic acid or commercial vinegar. These data suggest that treatment with acetic acid at various pH values affects peanut allergenicity and may explain the low prevalence of peanut allergy in Korea.
Peanut allergy is one of the most common and severe IgE-mediated food reactions.1 However, reports of severe anaphylaxis and fatalities from peanuts in Asia are relatively rare.2 In addition, there are differences in the prevalence and severity of peanut allergy in different parts of the world.1,2 Many genetic and environmental factors could be responsible for the epidemiologic characteristics of peanut allergy.3 Regional dietary habits and cooking methods also play a role in the prevalence of specific food allergies in various countries, as differences in the methods of peanut preparation affect their final allergenicity.1,4 For example, the amount of personal peanut consumption in China and the United States are similar, but there is virtually no peanut allergy in China.5 This is likely because the Chinese predominantly eat boiled or fried peanuts, while Americans eat almost exclusively dry-roasted peanuts.5 The higher heat of dry roasting and the process of maturation and curing have been shown to increase the allergenicity of peanut proteins.4,6,7 Koreans occasionally eat pickled peanuts. In our previous study, bands corresponding to peanut allergens such as Ara h 1, Ara h 2, and Ara h 3, as well as the IgE-binding intensities to these allergens, were shown to change after treatment of the peanuts with vinegar.8 The concentration of acetic acid, which is a key ingredient of vinegar, typically ranges from 4% to 8% by volume for table vinegar and up to 18% for pickling vinegar.
To our knowledge, no previous studies have evaluated changes in food allergens under acidic conditions; therefore, we attempted to determine the effects of various pH conditions on major peanut allergens.
Sera from seven patients sensitized to peanuts were pooled. The patients had a peanut-specific IgE levels>15 kUA/L, as measured using the Pharmacia CAP system (Uppsala, Sweden). This cutoff value was considered to be predictive of clinical reactivity.9 Normal human sera were used as negative controls. After receiving informed consent, blood samples were obtained and the sera were frozen at -80℃ until use. This study was approved by the Samsung Medical Center Institutional Review Board.
Raw in-shell peanuts grown in Korea were obtained from the Korean Rural Development Administration.10 Peanut kernels were soaked overnight in commercial vinegar (pH 2.3), and in acetic acid solutions with pH values of 1.0, 3.0, or 5.0. The peanuts were then ground using a mortar and pestle until a smooth paste was achieved. The paste was defatted by washing with n-hexane and dried overnight at room temperature. Proteins were then extracted from the dried powder by agitating the samples in phosphate-buffered saline (PBS) for 2 hours at 4℃. Following centrifugation at 3,000×g for 20 minutes at 4℃, supernatants were collected, filtered (0.45 µm pore size), and lyophilized. Protein concentrations were then determined using a 2-D Quant kit (Amersham Biosciences, Piscataway, NJ, USA) and a microplate reader (Bio-Rad, Hercules, CA, USA). Table shows the protein concentration of each peanut sample. All extracts were stored at -80℃.
Extracts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; Tricine System; Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Extracts were then loaded into each well of a 96-well plate at 6 µg/well. Precision Plus Protein standards (Bio-Rad) were used as molecular weight markers to estimate protein size. The separated proteins were transferred from the gel to a polyvinylidene difluoride membrane (PVDF) using the iBlot™ Dry Blotting System (Invitrogen). The PVDF membranes were then blocked with 2% non-fat dried milk (NFDM) for 1 hour at room temperature, and then incubated overnight with the patients' pooled sera, which was diluted 1:10 in 2% NFDM. After washing with PBS-Tween (PBS-T), the membrane was incubated with biotin-labeled goat IgG-anti-human IgE (KPL, Gaithersburg, MD, USA), which was diluted 1:4,000 in 2% NFDM, for 1 hour at room temperature. After rinsing with PBS-T, the membrane was incubated with NeutrAvidin-HRP (Pierce Chemical Co., Rockford, IL, USA) for 30 minutes at room temperature. After washing with PBS-T, the membrane was reacted with Amersham ECL reagent (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) for 2 minutes. The blotted membrane was then exposed to a high performance chemiluminescence film (GE Healthcare Limited, Buckinghamshire, UK) and the film was developed. The density of SDS bands and immunoblots were compared using a reflective densitometer (Bio-Rad) and Quantity One software (Bio-Rad).
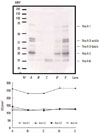
Almost all of the peanut allergens were altered after incubation in the acidic solutions (Fig. 1). The density of Ara h 1 was slightly reduced in samples treated with pH 3.0 or 5.0 acetic acid, and was completely undetected in samples treated at pH 1.0 acetic acid or commercial vinegar (pH 2.3). Ara h 2 remained largely unchanged at pH 5.0, but decreased as the soaking solutions became more acidic. Ara h 3 and Ara h 6 were unaltered by treatment with pH 3.0 or 5.0 acetic acid, but appeared as a very thick, strong band in samples treated with pH 1.0 acetic acid or commercial vinegar (pH 2.3). Bands between 50 and 38 kDa were significantly enhanced. This might be due to rearrangement of Ara h 3 after treatment with a strong acid, or aggregation of non-allergenic proteins.
The IgE-binding intensities to major peanut allergens, such as Ara h 1, Ara h 2, and Ara h 3, were significantly reduced at pH 1.0 and 2.3, although there was no substantial change at pH 3.0 or 5.0 when compared with raw peanuts (Fig. 2). The IgE-binding intensities to Ara h 1 were also reduced by exposure to pH 1.0 and commercial vinegar, which corresponded to the reduced quantities of Ara h 1. IgE-binding to Ara h 2 disappeared at pH 1.0 and after treatment with commercial vinegar. The IgE-binding intensities to Ara h 3 were reduced in samples treated at pH 1.0 and 2.3, despite the increased quantity of this protein (Fig. 1).
The prevalence of peanut allergy in Korea is unknown; however, similar to other Asian countries, it appears to be low, which differs from the incidence in Western nations.11,12 Although the exact cause of this low prevalence remains unclear, one possible explanation is differences in eating habits, which can affect food allergenicity. The allergenicity of peanut proteins can be altered by different cooking methods.4,13 It is difficult to predict the physical and chemical changes which occur in cooked food, because food contains very complicated organic compounds. The most important finding of this study was that treatment of peanuts with vinegar affected their allergenicity, and that such changes in allergenicity differed with pH. These findings correspond with those of previous studies in which different cooking methods resulted in changes in the final allergenicity of peanuts,4,6,7 suggesting that treatment of peanuts with vinegar could lead to regional differences in the prevalence and severity of peanut allergy.
Nine allergens have been reported in peanuts, and Ara h 1, Ara h 2, and Ara h 3 are believed to be the major allergens. Of these, the importance of Ara h 2 in clinical peanut allergy has been suggested in previous studies.14-19 A correlation between clinical severity and the presence of low concentrations of Ara h 2 and higher levels of Ara h 1 and 3 was reported to indicate a greater potency of Ara h 2.16 Component-resolved diagnostics also revealed Ara h 2 as the most important component for accurate discrimination of subjects with peanut allergy vs. peanut-tolerant subjects.20 In this study, IgE reactivities toward Ara h 1, Ara h 2, and Ara h 3 were decreased after exposure of peanuts to acidic solutions, and the reduction in IgE reactivity to Ara h 2 was marked. While Ara h 2 has a greater resistance to proteolytic digestion and heat/chemical treatment, our data suggest that Ara h 2 was degraded in acidic conditions with a subsequent reduction in IgE binding.
In conclusion, the allergenicity of major peanut allergens is altered by acid treatment. Further research is needed to investigate changes in the allergenicity of major food allergens according to the various cooking methods used in each region.
Figures and Tables
Fig. 1
SDS-PAGE analysis of peanut proteins at various pH values (up) and their optical density (down). Raw peanuts (lane A), peanuts after commercial vinegar treatment at pH 2.3 (lane B), and peanuts after treatment with pH 1.0, 3.0, and 5.0 acetic acid solutions (lanes C, D, and E, respectively) are shown. Molecular weight standards are shown in lane M.

Fig. 2
Detection of anti-peanut-specific IgE at various pH values (up) and their optical density (down) by immunoblotting. Raw peanuts (lane A), peanuts after commercial vinegar treatment at pH 2.3 (lane B), and peanuts after treatment with pH 1.0, 3.0, and 5.0 acetic acid solutions (lanes C, D, and E, respectively) are shown. Molecular weight standards are shown in lane M.
ACKNOWLEDGMENTS
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD; KRF-2006-214-C00099).
References
1. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004. 113:805–819.
2. Piromrat K, Chinratanapisit S, Trathong S. Anaphylaxis in an emergency department: a 2-year study in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2008. 26:121–128.
3. Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007. 120:491–503.
4. Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, Sampson HA. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001. 107:1077–1081.
5. Hill DJ, Hosking CS, Heine RG. Clinical spectrum of food allergy in children in Australia and South-East Asia: identification and targets for treatment. Ann Med. 1999. 31:272–281.
6. Chung SY, Butts CL, Maleki SJ, Champagne ET. Linking peanut allergenicity to the processes of maturation, curing, and roasting. J Agric Food Chem. 2003. 51:4273–4277.
7. Maleki SJ, Viquez O, Jacks T, Dodo H, Champagne ET, Chung SY, Landry SJ. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol. 2003. 112:190–195.
8. Ahn YH, Yeo JS, Lee JY, Han YS, Ahn KM, Lee SI. Effects of cooking methods on peanut allergenicity. Pediatr Allergy Respir Dis. 2009. 19:233–240.
9. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001. 107:891–896.
10. Koppelman SJ, Vlooswijk RA, Knippels LM, Hessing M, Knol EF, van Reijsen FC, Bruijnzeel-Koomen CA. Quantification of major peanut allergens Ara h 1 and Ara h 2 in the peanut varieties Runner, Spanish, Virginia, and Valencia, bred in different parts of the world. Allergy. 2001. 56:132–137.
11. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, Ma S, Lee BW. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010. 126:324–331. 331.e1–331.e7.
12. Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009. 20:339–346.
13. Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000. 106:763–768.
14. Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004. 34:583–590.
15. Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, Ogier V, Petit N, Proust B, Moneret-Vautrin DA, Burks AW, Bihain B, Sampson HA, Kanny G. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006. 118:250–256.
16. Peeters KA, Koppelman SJ, van Hoffen E, van der Tas CW, den Hartog Jager CF, Penninks AH, Hefle SL, Bruijnzeel-Koomen CA, Knol EF, Knulst AC. Does skin prick test reactivity to purified allergens correlate with clinical severity of peanut allergy? Clin Exp Allergy. 2007. 37:108–115.
17. McDermott RA, Porterfield HS, El Mezayen R, Burks AW, Pons L, Schlichting DG, Solomon B, Redzic JS, Harbeck RJ, Duncan MW, Hansen KC, Dreskin SC. Contribution of Ara h 2 to peanut-specific, immunoglobulin E-mediated, cell activation. Clin Exp Allergy. 2007. 37:752–763.
18. Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009. 39:1277–1285.
19. Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009. 39:1099–1108.
20. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, Härlin A, Woodcock A, Ahlstedt S, Custovic A. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010. 125:191–197.e1-13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download