Abstract
Purpose
Probiotic bacteria can induce immune regulation or immune tolerance in allergic diseases. The underlying mechanisms have been recently investigated, but are still unclear. The aim of this study was to evaluate the protective effects of the probiotic Lactobacillus rhamnosus (Lcr35) in a mouse model of asthma and to identify its mechanism of action.
Methods
Lcr35 was administered daily by the oral route at a dosage of 1×109 CFU/mouse in BALB/c mice for 7 days before the first sensitization. Clinical parameters and regulatory T (Treg) cells were examined. The role of CD4+CD25+Foxp3+ Treg cells was analyzed using a Treg cell-depleting anti-CD25 monoclonal antibody (mAb).
Results
Airway hyperresponsiveness, total IgE production, pulmonary eosinophilic inflammation, and splenic lymphocyte proliferation were suppressed after Lcr35 treatment. Th1 (IFN-γ) and Th2 (IL-4, IL-5, and IL-13) cytokines in the serum were suppressed, and the percentage of CD4+CD25+Foxp3+ Treg cells in the spleen was significantly increased in the Lcr35 treatment group. Anti-CD25 mAb administration abolished the protective effects of Lcr35, indicating that CD4+ CD25+Foxp3+ Treg cells are essential in mediating the activity of Lcr35.
Probiotics are non-pathogenic microorganisms that confer a health benefit on the host1 by improving the balance of the intestinal microflora and possibly by augmenting host defenses. Several studies have characterized the ability of probiotic strains to alter cytokine production in the gut and associated lymphoid tissue, demonstrating immunomodulatory effects on some allergic diseases.2-4 Successful probiotic treatments in animal models of asthma have recently been described,5,6 and we have reported that oral Lactobacillus rhamnosus (Lcr35) treatment prior to allergic sensitization can attenuate airway inflammation and hyperreactivity in a mouse model of allergic airway inflammation.7 However, the beneficial effects of probiotics on the development of allergic asthma and allergic rhinitis in human clinical trials remains controversial.8,9 Despite conflicting results, it is generally thought that the immunomodulatory effect of probiotics on allergic diseases is a promising area of research.10,11
A number of studies have suggested that the immunosuppressive cytokines interleukin-10 (IL-10) and transforming growth factor (TGF)-β along with regulatory T (Treg) cells are the main mechanisms by which probiotics suppress allergic inflammation.2,5,12 Nevertheless, there have been few mechanistic studies of probiotic activity in allergic disease. In the present study, we aimed to confirm the involvement of Treg cells in the protective effect of Lcr35 in a mouse model of allergic asthma.
Female BALB/c mice weighing 20-25 g (4 weeks old) were purchased from Orient Bio (Orient Bio Inc., Seongnam, Korea) and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Asan Medical Center and Ulsan University College of Medicine.
The Lcr35 used in this study was obtained from the Lyocentre® laboratory (Aurillac, France) and was cultured in sterile Lactobacillus MRS broth (Difco, Sparks, MD, USA) at 37℃ for 24 hours. After cultivation, cells were collected by centrifugation at 5,000×g for 10 minutes, washed twice with phosphate buffered saline (PBS), and lyophilized. The lyophilized powder was reconstituted with sterile saline before use.
Six-week-old female BALB/c mice (N=7 per group) were sensitized by intraperitoneal (i.p.) administration of an ovalbumin (OVA; 10 µg, grade V; Sigma Chemical Co., St. Louis, MO, USA) and alum (2.25 mg; Imject®, Pierce, Rockford, IL, USA) mixture. One week after the first sensitization, the mixture was administered a second time. Seven days later, the mice inhaled 1% OVA via an ultrasonic sprayer (Nescosonic UN-511; Alfresa, Osaka, Japan) for 30 minutes daily for three successive days (OVA challenge). The mice received Lcr35 (1×109 colony-forming units/600 µL/mouse/day) orally from one week before primary sensitization to the endpoint of the study. Negative controls received only saline instead of OVA at both sensitizations and airway challenge. Positive controls received nothing more after OVA sensitization.
Airway hyperresponsiveness (AHR) in response to inhaled methacholine (MeCh; Sigma Chemical Co.), administered 24 hours after OVA challenge, was measured in conscious, unrestrained mice using a barometric whole-body plethysmograph (Buxco; EMKA Technologies, Paris, France). Briefly, mice were placed in a whole-body chamber, and basal readings were obtained for 3 minutes and averaged. Aerosolized saline followed by 5-50 mg/mL MeCh were inhaled for 3 minutes after each MeCh inhalation.
Treg cells were depleted using anti-CD25 monoclonal antibody (mAb). Briefly, mice received 250 µg of rat anti-mouse CD25 mAb (clone PC61; eBioscience, San Diego, CA, USA) i.p. in 400 µL of normal saline one day before 1% OVA challenge. Control mice were injected with 250 µg of rat IgG1 (Sigma Chemical Co.).
After measurement of AHR, the mice were anesthetized by i.p. administration of ketamine-xylazine, and the trachea was immediately exposed. The airways were lavaged through a tracheal cannula, two times with 1-mL aliquots of pyrogen-free saline warmed to 37℃. The recovered lavage fluid was pooled, and the cells were collected by centrifugation (5,000 rpm, 4℃, 5 minutes) and resuspended in 100 mL of cold PBS. The cells were stained with trypan blue to determine viability, and total nucleated cells were counted using a hemocytometer.
For differential BAL cell counts, cytospin preparations were made and stained with Diff-Quik (Sysmex, Takatsukadai, Japan). After the samples were coded, all cytospin preparations were evaluated by one observer using an oil immersion microscope (magnification, ×1,000). At least 200 cells were counted per preparation, and the absolute number of each cell type was calculated.
Serum was obtained from blood taken during exsanguination of the mice after airway measurement, and 100 µL (1/10 dilution in carbonate-bicarbonate buffer) were added to each well of a 96-well plate. An IgE-specific enzyme linked immunosorbent assay (ELISA) was used to quantitate total IgE in the serum, using matching antibody pairs (eBioscience) according to the manufacturer's instructions. For the ELISA, 96-well plates were first coated overnight with rat anti-mouse IgE (10 µL in 100 µL of PBS; PharMingen, San Diego, CA, USA), rat anti-mouse IgG1 (20 µg in 100 µL of PBS; PharMingen), or rat anti-mouse IgG2a (20 µg in 100 µL of PBS; PharMingen). The remaining binding sites were blocked, and the plates were incubated with 100 µL of diluted serum (1:5 for IgE, 1:10 for IgG1 or IgG2a). After the plate was washed, each of the following was sequentially added, incubated, and removed by washing: OVA (1 µg/100 µL), peroxidase-labeled rabbit anti-OVA Ig (240 ng/100 µL, PharMingen), and 3,3,5,5-tetramethylbenzidine solution (Sigma Chemical Co.). The optical density was measured at 450 nm, and the Ig level was determined relative to that of a reference pool of serum from OVA-sensitized BALB/c mice (assigned a value of 100 experimental units/mL). Determinations were performed in duplicate.
Commercial preparations of paired antibodies and protein standards for measurements of mouse IL-4, IL-5, IL-13, and IFN-γ (eBioscience) in sera were used to develop ELISAs according to the manufacturer's instructions. Determinations were performed in duplicate.
After BAL, the mouse spleen was resected. Mouse splenocytes were separated on a Histopaque (Sigma Chemical Co.) gradient, and the collected cells were washed with PBS. RBCs were lysed by gently mixing the cells with 3.6 mL of 0.24% NaCl for 20 seconds, followed by the quick addition of 0.3 mL of 8.7% NaCl and further dilution with PBS. The pellet was suspended in Iscove's Modified Dulbecco's Medium (IMDM), and stored overnight at 4℃. The next morning, the cells were centrifuged at 4℃, suspended in cold PBS, stained with trypan blue, and counted using a hemocytometer.
Splenic T cells were cultured in IMDM supplemented with 25 mM HEPES, 10% (v/v) heat inactivated fetal bovine serum (FBS), 60 mg/L (100 U/mL) penicillin, 100 mg/L streptomycin, and 0.29 g/L L-glutamine. Splenic T cells were adjusted to 1×105 cells/200 µL/well, transferred to 96-well plates, and incubated at 37℃ in a humidified 5% CO2 incubator for 72 hours. The cells were stimulated with OVA treatment (100 µg/mL) for 72 hours. At 12 hours before the end of the incubation, 1 µCi of [3H]-thymidine was added to each well. The cells were harvested onto a glass microfiber filter (Simport, Beloeil, Canada), and radioactivity was measured in a liquid scintillation counter. The incorporation during the last 12 hours of culture (counts per minute) was used as an index of proliferation. All cultures were tested in triplicate.
Mouse Treg cells were collected from the spleen and analyzed for CD4+CD25+Foxp3+ expression using a mouse Treg cell staining kit containing FITC-labeled anti-CD4, APC-labeled anti-CD25, and PE-labeled anti-Foxp3 (eBioscience) according to the manufacturer's instructions. Briefly, prepared cells (1×106) were washed by centrifugation with cold PBS, resuspended in 1 mL of fixation/permeabilization solution, and incubated in the dark at 4℃ for 30-60 minutes. The cells were washed once with 2 mL of permeabilization buffer, collected by centrifugation, resuspended in 20 mL of blocking agent with 2% (2 mL) normal rat serum in permeabilization buffer, and incubated at 4℃ for 15 minutes. Next, 20 mL of fluorochrome-conjugated antibody or isotype control in permeabilization buffer were added, followed by incubation in the dark at 4℃ for 30 minutes. Finally, the cells were washed with 2 mL of permeabilization buffer, resuspended in flow cytometry buffer (PBS with 2% FBS), and analyzed by flow cytometry using a FACSCalibur with CellQuest software (BD Biosciences, Mountain View, CA, USA). Determinations were performed in duplicate.
For the histological evaluation of lung tissue, the left lung of each mouse was embedded in paraffin, sectioned to a thickness of 5 µm, and stained with hematoxylin and eosin (H&E) to assess eosinophilic infiltration. Inflammation was scored by two independent, blinded investigators. The degree of peribronchial and perivascular inflammation was evaluated on a subjective scale of 0-3, as described elsewhere.13 Cellular infiltration in five randomly selected fields was assessed under a Zeiss Axiophot microscope (magnification, ×100; Carl Zeiss, Inc., Thornwood, NY, USA).
We analyzed the association between groups and positive controls using the Mann-Whitney test. Data are expressed as means±standard error. All statistical analyses were performed using SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). A P value<0.05 indicated significance.
Lcr35 administration to allergic mice significantly suppressed AHR, to the level of the negative control group at the maximum MeCh dose (negative control: 4.72±2.19; Lcr35-treated: 4.75±1.56; positive control: 9.00±2.87; P<0.01) (Fig. 1A).
Lcr35-treated mice exhibited a significant suppression of BAL fluid total cell count compared with positive controls (Lcr35-treated: 4.77×105 cells/mL; positive control: 8.29×105 cells/mL; P<0.05). Oral administration of Lcr35 significantly reduced the number of eosinophils in BAL fluid compared with positive controls (Lcr35-treated: 3.79±0.29%; positive control: 13.60±3.00%; P<0.05) (Fig. 1B).
Lung inflammation after OVA inhalation was significantly reduced by oral administration of Lcr35 (Lcr35-treated: 2.16±0.55; positive control: 0.92±0.52; P<0.05) (Fig. 1C). Examination of the lung tissue of positive control mice revealed peribronchial and perivascular cellular infiltrates; Lcr35 treatment produced a marked decrease in both cellular infiltration and inflammatory changes, as determined by histopathology (Fig. 1D). Thus, Lcr35 treatment inhibited allergen-induced pulmonary inflammation, including the influx of eosinophils.
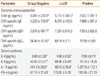
The administration of Lcr35 significantly reduced the total IgE level compared with that of the positive control, but had no significant effect on OVA-specific IgE, IgG1, or IgG2a (Table). Serum IL-4, -5, and -13 levels were significantly suppressed by orally administered Lcr35. The IFN-γ level was also decreased in Lcr35-treated mice, but the difference was not significant (Table). These results suggest that Lcr35 treatment suppresses Th2-dependent cytokines. OVA-induced cell proliferation was significantly lower in Lcr35-treated mice than in positive control mice (Fig. 2). Thus, Lcr35 effectively inhibited the OVA-specific splenic T cell response.
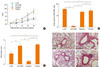
Lcr35 treatment led to a significant increase in CD4+CD25+Foxp3+ Treg cells, compared with the percentage in positive control mice (Lcr35-treated: 17.60±0.49%; positive control: 15.20±0.44%; P<0.05) (Fig. 3B). This suggests that Lcr35-induced attenuation of allergic responses in the mouse model of asthma is associated with an increased CD4+CD25+Foxp3+ Treg cell population. To confirm the involvement of CD4+CD25+Foxp3+ Treg cells, Treg cell depletion was achieved by anti-CD25 mAb treatment. Anti-CD25 mAb treatment before OVA challenge significantly increased AHR at the maximum MeCh dose, compared with AHR in Lcr35-treated mice (anti-CD25-treated: 8.16±1.63; Lcr35-treated: 3.30±0.57; P<0.05) (Fig. 4A). The isotype control rat IgG1 had no effect on AHR (data not shown). Anti-CD25 treatment also significantly decreased the population of CD4+CD25+Foxp3+ Treg cells in Lcr35-treated mice (anti-CD25 treated: 1.00±0.08%; Lcr35-treated: 15.53±0.10%; P<0.05) (Fig. 4B). These results suggest that CD4+CD25+Foxp3+ Treg cells are involved in the suppression of AHR mediated by oral administration of Lcr35.
The pulmonary inflammation score was also significantly higher in anti-CD25 mAb-treated Lcr35 mice compared with Lcr35-treated mice that did not receive anti-CD25 mAb (anti-CD25 treated: 2.28±0.19; Lcr35-treated 1.32±0.20; P<0.01) (Fig. 4C). The histopathology revealed enhanced eosinophilic inflammation in the lungs of anti-CD25-treated Lcr35 mice compared with anti-CD25 mAb-non-treated Lcr35 mice (Fig. 4D). The data indicate that administration of anti-CD25 mAb reverses the effect of oral Lcr35 treatment on lung inflammation and that Treg cells mediate the effect of Lcr35.
This study examined the effect of oral Lcr35 on allergic asthma in a mouse model. The major finding was that Lcr35 suppresses allergic parameters, including AHR, airway inflammation, and total IgE, by regulating the activity of CD4+CD25+Foxp3+ Treg cells. In addition, these effects were reversed by administration of anti-CD25 mAb.
The effect of probiotics on Th1 cytokines is controversial, as probiotics have been shown to promote, inhibit, and have no effect on Th1 cytokine production.14-16 In our study, serum levels of Th2 cytokines were reduced by oral Lcr35 treatment, whereas Th1 cytokine levels were not significantly reduced. We did not determine cytokine levels in target organs or BAL fluid; this is a limitation of this study.
To test our hypothesis, CD4+CD25+Foxp3+ Treg cell numbers in the spleen were determined. Treg cell numbers were significantly higher after Lcr35 treatment. Exacerbation readouts of asthma after Treg depletion demonstrated the importance of Treg cells in the protective effect of Lcr35. This suggests that Treg cells may be involved in the protection conferred by oral Lcr35 in the mouse model of asthma. However, other potential mechanisms such as immunosuppressive cytokines (IL-10 and TGF-β) were not examined. To our knowledge, this is the first study to identify the mechanism of action of probiotics using depletion of CD4+CD25+Foxp3+ Treg cells in a mouse model of asthma.
Some reports have suggested that the involvement of thymus-derived natural Treg cells expressing Foxp3 and CD25 in tolerance to allergens is unlikely, and the expression of Foxp3 and CD25 on Treg cells remains controversial.10 Further studies are needed to confirm the mechanism underlying the protective effect of Lcr35.
Many studies have attempted to identify the mechanism of action of probiotics in allergic diseases.2-4 Several have implicated Treg cells in the activity of probiotics,2,17 but these studies did not investigate changes in the inflammatory response after Treg cell depletion. Our study is important in that it confirms previous hypotheses that Treg cells contribute to the protective effect of probiotics.
Oral administration of Lcr35 completely blocked OVA-specific proliferation of splenic T cells in the present study. This suggests that Lcr35 regulates the systemic immune response in OVA-sensitized and -challenged mice. Oral administration of probiotics induces regulatory dendritic cells, which in turn promote the generation of CD4+Foxp3+ Treg cells in mesenteric lymph nodes.2 Dendritic cells can directly present antigens from commensal bacteria to mesenteric lymph nodes and interact with T and B cells to maintain a non-inflammatory immune response.3,4
In contrast to the massive eosinophilic infiltration in the peribronchial and perivascular areas of positive control mice, animals treated with Lcr35 showed significantly less eosinophilic inflammation. This was coincident with decreased production of the Th2 cell-derived cytokines IL-4, IL-5, and IL-13 in the Lcr35 treatment group.
There is strong evidence implicating CD4+CD25+ Treg cells in controlling allergic diseases. For example, the transfer of CD4+CD25+ Treg cells to sensitized mice reduced AHR, eosinophilic inflammation, and Th2 cytokine induction in the lung, in an IL-10-dependent manner.18,19 It is widely believed that administration of an anti-CD25 mAb results in the rapid and efficient depletion of CD4+CD25+ Treg cells, and this has been confirmed by secondary staining with a mAb directed against a different CD25 epitope.20,21 Previous studies have shown that the number of CD4+CD25+ Treg cells was significantly decreased within 3 days of anti-CD25 mAb treatment in vivo, but had recovered to normal levels by day 10.22,23 Therefore, in this study, we administered anti-CD25 mAb one day before OVA challenge. As expected, the anti-allergic effects of Lcr35 disappeared, and the CD4+CD25+Foxp3+ Treg cell population was significantly reduced. This demonstrates that the beneficial effect of Lcr35 on allergic asthma in the mice was mediated by CD4+CD25+Foxp3+ Treg cells.
In conclusion, we demonstrated that the suppression of allergic responses and immunomodulation by the probiotic Lcr35 in a mouse model of asthma was mediated by the activity of CD4+CD25+Foxp3+ Treg cells.
Figures and Tables
Fig. 1
Effect of oral Lcr35 treatment on allergic asthma in a mouse model. (A) Airway hyperresponsiveness; (B) Eosinophil proportion in bronchoalveolar lavage fluid; (C) Peribronchial and perivascular lung inflammation score; (D) Lung pathology. *P<0.05 and **P<0.01. Negative control: Saline (i.p.)/saline challenge by inhalation. Lcr35-treated: OVA (i.p.)/OVA challenge by inhalation+Lcr35 (oral administration). Positive control: OVA (i.p.)/OVA challenge by inhalation.
Lcr35, Lactobacillus rhamnosus; i.p., intraperitoneal; OVA, ovalbumin.
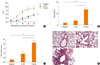
Fig. 2
Effect of oral Lcr35 treatment on the suppression of OVA-specific splenic T cell proliferation in a mouse model. *P<0.05 and **P<0.01. Negative control: Saline (i.p.)/saline challenge by inhalation. Lcr35-treated: OVA (i.p.)/OVA challenge by inhalation+Lcr35 (oral administration). Positive control: OVA (i.p.)/OVA challenge by inhalation.
Lcr35, Lactobacillus rhamnosus; OVA, ovalbumin; i.p., intraperitoneal.

Fig. 3
Effect of oral Lcr35 treatment on the CD4+CD25+Foxp3+ Treg population in a mouse model. (A) Flow cytometric analysis of T cells in the mouse spleen. Plots show cells expressing CD4, CD25, and intracellular Foxp3. Numbers inside the histograms indicate the percentage of cells; (B) Relative proportion of CD4+CD25+Foxp3+ Treg cells among total splenic T cells after Lcr35 treatment in comparison with cells obtained from the positive control (Group 3). *P<0.05. Negative control: Saline (i.p.)/saline challenge by inhalation. Lcr35-treated: OVA (i.p.)/OVA challenge by inhalation+Lcr35 (oral administration). Positive control: OVA (i.p.)/OVA challenge by inhalation.
Lcr35, Lactobacillus rhamnosus; i.p., intraperitoneal; OVA, ovalbumin.

Fig. 4
Effect of anti-CD25 mAb treatment on allergic asthma in a mouse model after oral Lcr35-treatment. (A) Airway hyperresponsiveness; (B) Relative proportion of CD4+CD25+Foxp3+ Treg cells among total splenic T cells; (C) Comparison of pulmonary inflammation scores; (D) Lung pathology (H&E stain; ×100). *P<0.05. Lcr35-treated: OVA (i.p.)/OVA challenge by inhalation+Lcr35 (oral administration). Anti-CD25: OVA (i.p.)/OVA challenge by inhalation+Lcr35 (oral administration)+anti-CD25 Ab (i.p.). Negative control: Saline (i.p.)/saline challenge by inhalation. Positive control: OVA (i.p.)/OVA challenge by inhalation.
Lcr35, Lactobacillus rhamnosus; OVA, ovalbumin; i.p., intraperitoneal.
ACKNOWLEDGMENTS
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (NRF-2007-313-E00268).
References
1. Marteau P. Probiotics, prebiotics, synbiotics: ecological treatment for inflammatory bowel disease? Gut. 2006. 55:1692–1693.
2. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010. 107:2159–2164.
3. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004. 303:1662–1665.
4. Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000. 191:435–444.
5. Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007. 37:498–505.
6. Hong HJ, Kim E, Cho D, Kim TS. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. J Clin Immunol. 2010. 30:449–458.
7. Yu J, Jang SO, Kim BJ, Song YH, Kwon JW, Kang MJ, Choi WA, Jung HD, Hong SJ. The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol Res. 2010. 2:199–205.
8. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001. 357:1076–1079.
9. Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002. 57:243–246.
10. Shida K, Takahashi R, Iwadate E, Takamizawa K, Yasui H, Sato T, Habu S, Hachimura S, Kaminogawa S. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exp Allergy. 2002. 32:563–570.
11. Pochard P, Gosset P, Grangette C, Andre C, Tonnel AB, Pestel J, Mercenier A. Lactic acid bacteria inhibit Th2 cytokine production by mononuclear cells from allergic patients. J Allergy Clin Immunol. 2002. 110:617–623.
12. Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005. 115:1260–1267.
13. Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000. 30:79–85.
14. Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009. 44:26–46.
15. Torii A, Torii S, Fujiwara S, Tanaka H, Inagaki N, Nagai H. Lactobacillus acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol Int. 2007. 56:293–301.
16. Segawa S, Nakakita Y, Takata Y, Wakita Y, Kaneko T, Kaneda H, Watari J, Yasui H. Effect of oral administration of heat-killed Lactobacillus brevis SBC8803 on total and ovalbumin-specific immunoglobulin E production through the improvement of Th1/Th2 balance. Int J Food Microbiol. 2008. 121:1–10.
17. Mastrangeli G, Corinti S, Butteroni C, Afferni C, Bonura A, Boirivant M, Colombo P, Di Felice G. Effects of live and inactivated VSL#3 probiotic preparations in the modulation of in vitro and in vivo allergen-induced Th2 responses. Int Arch Allergy Immunol. 2009. 150:133–143.
18. Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005. 202:1539–1547.
19. Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008. 122:617–624.e6.
20. McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002. 168:5979–5983.
21. Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004. 172:4686–4690.
22. Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005. 174:4525–4534.
23. Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006. 176:3301–3305.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download