Abstract
Purpose
We investigated whether particular HLA-DRB1 polymorphisms contribute to egg allergy development in Korean children with atopic dermatitis (AD).
Methods
HLA-DRB1 alleles were determined by PCR-sequence-specific oligonucleotide (SSO) and PCR-single-strand conformation polymorphism (SSCP) methods in 185 patients with AD and 109 normal control (NC) subjects. AD patients were divided into two groups: 1) AD with egg allergy, consisting of 96 patients with egg allergies as determined by egg-specific immunoglobulin E (IgE) reactivity; and 2) AD without egg allergy, consisting of 89 patients without egg allergies. HLA-DRB1 alleles were classified into functional groups (A, De, Dr, E, Q, R, a). HLA-DRB1 phenotype and functional group frequencies in the AD, AD with egg allergy, and AD without egg allergy groups were compared with those in the NC group.
Results
The frequency of DRB1*08:02 was decreased in the AD with egg allergy group compared with the AD without egg allergy group (2.1% vs. 10.1%, P=0.021), and DRB1*15:01 was increased in the AD with egg allergy group compared with the AD without egg allergy group (22.9% vs. 11.2%, P=0.036). However, significance was lost after Bonferroni correction. HLA-DRB1*11:01 had a significantly higher frequency in AD patients compared with NCs (12.4% vs. 1.8%, corrected P=0.048) and was regarded as a susceptibility factor associated with AD. DRB1*08:03 was decreased in AD patients compared with NCs (10.8% vs. 19.3%, P=0.043). HLA-DRB1 functional group 'a', which includes DRB1*15:01, seemed to be associated with the development of egg allergy in AD (P=0.033), but this result was not significant after Bonferroni correction.
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disorder associated with interactions between environmental and genetic factors. Food allergy is found in 35 to 45% of children with moderate to severe AD.1 Among various food allergens, hen's egg, particularly egg white, is one of the most common causes of food allergy in young children, and egg allergy is correlated with AD severity and the likelihood of additional food allergies.2,3 The risks for sensitization to aeroallergens4 and asthma5 are also increased in children with egg allergy.
Identifying the responsible gene in food allergy and AD would lead to identification of children who require early preventive intervention. Many studies have attempted to determine the genetic loci involved in allergic responses. Genes such as CD14,6,7 FOXP3,8 STAT6,9 SPINK5,10,11 interleukin (IL)-10,12 and KoreaIL-1313 have been studied in food allergy patients, but showed inconsistent results. One candidate gene that may be involved in food allergy is human leukocyte antigen (HLA), because it plays a major role in immune response regulation and is associated with predisposition to a large number of immunologically mediated diseases.
HLA class I molecules are known to influence apoptosis, an important regulatory function in inflammatory responses. Polymorphisms within this region may therefore be related to the duration and severity of inflammation.14 HLA class II molecules have extensive molecular polymorphisms, almost entirely confined to the peptide-binding groove. These polymorphisms determine which antigen-derived peptides are bound and presented to T cells via T cell receptors. In the presence of appropriate co-stimulatory signals, this results in the activation of the T cells recognizing the HLA-peptide complex. Antigen presentation to T cells by HLA molecules is a defining step in the development of antigen-specific immune responses.15 Certain HLA class II alleles (DR, DQ, DP) influence specific IgE responses to airborne pollens15-17 and house dust mite allergens.18,19 With regard to food allergies, HLA-DQ7 has been positively associated with cow's milk allergy.20 HLA-DR and DP molecules have been shown to restrict T cell recognition of peanut allergens,21 and HLA-B*07 and HLA-DRB1*11 are associated with nut allergy.
To our knowledge, little has been documented regarding the association between HLA class II polymorphisms and egg allergy in AD. The purpose of this study was to investigate whether HLA-DRB1 polymorphisms contribute to the development of egg allergy in Korean children with AD.
We enrolled 185 unrelated Korean children with AD who visited the pediatric clinic at Samsung Medical Center. AD was diagnosed according to the criteria of Hanifin and Rajka,23 and AD severity was assessed from 0 to 108 points based on the Six Area, Six Sign AD (SASSAD) severity score.24 AD patients were divided into two groups: AD with (n=96) and without (n=89) egg allergy. Egg allergy was confirmed when the egg white-specific IgE antibody level was >2 kU/L in children younger than 2 years of age, or >7 kU/L in children 2 years of age and older, according to the concentration associated with clinical reactions in more than 95%.25 As normal controls, 109 children with no personal or family history of allergic disease were recruited. Their sera were negative for six major food allergen (egg white, cow's milk, soy, peanut, wheat, and buckwheat)-specific IgE.
This study was approved by the Institutional Review Board at Samsung Medical Center, and informed consent was obtained from the parents of all subjects.
Serum samples were obtained from all participants and tested for specific IgE against six major common food allergens (egg white, cow's milk, soy, peanut, wheat, and buckwheat) using a CAP system fluorescent enzyme immunoassay (Pharmacia-Upjohn Diagnostics, Uppsala, Sweden), according to the manufacturer's instructions. Values greater than 0.35 kU/L were considered positive.
Phenotype frequencies of HLA-DRB1 alleles were compared among the AD with egg allergy, AD without egg allergy, and normal control groups. For comparison, the genomic DNA of normal controls was isolated from EDTA-anticoagulated peripheral blood samples using a LaboPass™ Blood Mini Kit (Cosmo genetech, Korea). To extract the genomic DNA of patients, a pH method with FTA cards (Whatman, Florham Park, NJ, USA) was used. Blood was applied to the cards, which were then washed with FTA purification reagent and rinsed with TE-1 buffer (10 mM Tris-HCI, 0.1 mM EDTA, pH 8).
HLA-DRB1 genotyping was performed by DNA typing methods using two steps. Low resolution HLA-DR typing was carried out by the PCR-sequence-specific oligonucleotide (SSO) method using a commercially available kit (RELI™ SSO HLA-DRB Test; Dynal Biotech Ltd., Bromborough, United Kingdom). For high resolution HLA-DRB1 typing, group-specific amplification and single-strand conformation polymorphism (SSCP) analyses were performed as described elsewhere.26
HLA-DRB1 molecules were classified into seven functional groups (A, De, Dr, E, Q, R, a),27 representing the polymorphic residues 70, 71, and 74 of the β chain located in pocket 4, which exert a major influence on peptide binding and its subsequent recognition by T cells (Table 1).28 The frequencies of HLA-DRB1 functional groups were compared among the AD with egg allergy, AD without egg allergy, and normal control groups. HLA-DRB1 alleles are described according to the recent 2010 HLA nomenclature system.29
Phenotype frequencies of HLA alleles in the AD with and without egg allergy groups and normal controls were compared using the chi-square test or Fisher's two-tailed exact test. For multiple allelic frequency comparisons, P values were corrected (Pc) by multiplying the total number of alleles observed at each HLA-DRB1 locus (Bonferroni correction): for DRB1 alleles, ×29, and for functional groups, ×7. Clinical data between different groups were compared using the chi-square test for categorical variables and Student t test for continuous variables. A P or Pc value of less than 0.05 was considered to indicate a statistically significant difference.
The 185 patients with AD in this study included 80 females and 105 males (57%) with a mean age of 24 months. Demographic data of the subjects are presented in Table 2. Fifty patients (27%) had a family history of allergic disease. The mean serum egg white-specific IgE level in the patients was 37.4±36.9 kU/L. The mean SASSAD score was 16.9±12.5. Among the 185 patients, 96 (51.9%) had egg allergy, and 89 (48.1%) did not. Between the AD with egg allergy and AD without egg allergy groups, there was no significant difference in sex (male 62.5% vs. 50.6%, P=0.07) or family history of allergic diseases (22.9% vs. 31.5%, P=0.24). However, the AD with egg allergy group had a higher total IgE level (2,074 vs. 92 kU/L, P<0.001), higher egg white-specific IgE level (37.4 vs. 0 kU/L, P<0.001), and higher SASSAD score (20.1 vs. 11.4, P<0.001). Those in the AD with egg allergy group were significantly younger than those in the AD without egg allergy group (19 vs. 29 months, P=0.004).
The 109 normal control subjects, including 40 females and 69 males (63%) with a mean age of 57 months, had no history of allergy and were negative for specific IgE against six common food allergens.
The HLA phenotype frequencies are given in Tables 3 and 4. Compared with the normal controls, the AD patients had lower frequencies of HLA-DRB1*08:03 (10.8% vs. 19.3%, P=0.043) and HLA-DRB1*13:02 (11.4% vs. 19.3%, P=0.061) (Table 3), but these differences were not significant after correction for multiple comparisons (Pc>0.05). The frequency of HLA-DRB1*11:01 differed significantly between the AD patients (12.4%) and normal controls (1.8%) (P=0.002, Pc=0.048, odds ratio [OR]=7.796, 95% confidence interval [CI]=1.775-32.883) and was considered to be a factor associated with AD susceptibility.
The frequencies of HLA-DRB1 phenotypes were analyzed in the AD with egg allergy and AD without egg allergy groups, and compared with the frequencies in the normal controls (Table 4). The HLA-DRB1*08:02 frequency was lower in the AD with egg allergy group compared with the AD without egg allergy group (2.1% vs. 10.1%, P=0.021) and was regarded as a weak protective factor against egg allergy development in AD. The HLA-DRB1*15:01 frequency was higher in the AD with egg allergy group compared with the AD without egg allergy group (22.9% vs. 11.2%, P=0.036) and was regarded as a weak susceptibility factor associated with egg allergy development in AD.
The HLA-DRB1*11:01 frequency was higher in the AD with and without egg allergy groups than in the normal controls (13.5% vs. 1.8%, P=0.001, OR=8.380, 95% CI=1.840-38.162; 11.2% vs. 1.8%, P=0.006, OR=6.772, 95% CI=1.443-31.772); this difference remained significant after Bonferroni correction (Pc=0.038). No significant difference in the HLA-DRB1*11:01 frequency was observed between the AD with egg allergy and AD without egg allergy groups. Thus, HLA-DRB1*11:01 may be associated with AD susceptibility, rather than egg allergy.
The AD with egg allergy group exhibited a significantly higher frequency of functional group 'a' compared with the AD without egg allergy and normal control groups (P=0.033, OR=1.977, 95% CI=1.051-3.718; Table 5). The trend toward an association between the development of egg allergy in AD and the functional group 'a' was not significant after Bonferroni correction (P=0.24).
An association between AD and HLA has been reported by several investigators, but few studies have shown a significant association between AD and HLA molecules. Ozawa et al.30 reported that patients with AD, bronchial asthma, and allergic rhinitis showed a significantly increased frequency of both HLA-B12 and B40, although no overall difference in HLA antigen frequencies was seen in patients with AD only. Schultz Larsen and Grunnet31 were also unable to demonstrate a significant association between AD and the frequency of HLA-A, B, or C antigens. The association of HLA class I gene polymorphisms with AD has been reported in Korean patients.32 HLA-A24 allele frequency was increased in AD, but statistical significance was lost after Bonferroni correction. Saeki et al.33 reported that the frequency of HLA-DRB1*13:02 was increased in patients with severe AD and high serum IgE compared with controls, showing a susceptibility tendency associated with AD. However, this also did not remain significant after Bonferroni correction. Svejgaard et al.34 reported that the frequency of the HLA-DR7 antigen was decreased in patients with AD, but the significance of this deviation disappeared when P values were corrected.
In the present study, HLA-DRB1*11:01 was significantly increased in Korean children with AD compared with controls (P=0.002, Pc=0.048, OR=7.796). To our knowledge, this is the first report implicating a specific HLA-DRB1 allele in AD susceptibility. The exact mechanism underlying the effect of HLA class II polymorphisms on AD development is not fully understood; however, it seems that HLA class II alleles play key roles in antigen presentation to CD4+ T lymphocytes via T cell receptors and thus influence specific IgE response to several allergens.35 Therefore, these alleles are excellent candidates for determination of specific allergic responses.
Few studies have considered immunological responses to food. Camponeschi et al.20 found a relationship between HLA-DQ7 and cow's milk allergy; however, as the control group was not atopic, it is unclear whether the association was related to atopy or milk allergy. Boehncke et al.36 evaluated patients with pollen allergy and those with pollen and food allergies compared with healthy controls. Although they found some associations with specific alleles, the associations with food allergies such as carrot and peanut were explained primarily by susceptibility to pollen allergy. With regard to peanut allergy, HLA-DR and DP molecules restrict T cell recognition of peanut allergens.21 Nevertheless, no association was reported in a cohort of sibling pairs.37 HLA-B*07 and HLA-DRB1*11 were investigated as predisposing factors in nut allergy, but there was no statistical significance.22 The synergistic effect of anti-HLA class II monoclonal antibodies on proliferative responses of T cells to ovalbumin in egg-sensitized patients was investigated.38 Proliferation of T cells from AD patients with a low immune reaction was restored by anti-HLA-DQ monoclonal antibodies, and the proliferation of T cells from other patients was inhibited by anti-HLA-DP monoclonal antibodies. These results suggest that a component of the T cell repertoire that is reactive with ovalbumin in egg-sensitized patients may be restricted by HLA-DP molecules.
In the present study, we found no statistically significant association between HLA-DRB1 and egg allergy. HLA-DRB1*15:01 and HLA-DRB1*08:02 were regarded as susceptibility (P=0.036) and protective factors (P=0.021), respectively, for the development of egg allergy in AD. However, neither remained significant after P values were corrected. As many egg allergy patients in our study were also sensitized to other foods, we selected the patients who were allergic to egg alone (n=22) and compared the HLA-DRB1 phenotype frequency between these patients and normal controls. Again, we failed to find alleles significantly associated with egg allergy (data not shown).
Thus far, nucleotide sequence polymorphisms have been identified in at least 762 distinct HLA-DRB1 alleles.29 However, nucleotide differences do not account well for the observed variations in the associations with disease susceptibility or resistance. Differences in amino acid sequences are more relevant, because a single substitution at a critical residue of the HLA binding groove can alter the binding of antigenic peptides and totally change the nature of the immune response. HLA-DRB1 proteins contain subregions (pockets) in the DR binding groove which exert a major influence on peptide binding and its subsequent recognition by T cells.28 In particular, the polymorphic residues 70, 71, and 74 of the β chain located in pocket 4 play a critical role in the recognition of the HLA-DR/peptide complex by the CD4+ helper T cell.39 HLA-DRB1 alleles are categorized into seven DR restrictive supertypes (A, De, Dr, E, Q, R, and a) on the basis of physicochemical characteristics, especially charge, of the polymorphic residues. This novel categorization of DR alleles on the basis of function allows the prediction of specific sequence motifs within pocket 4 of the HLA-DR peptide-binding groove that are associated with autoimmune and infectious disease susceptibility.27 According to a Spanish study, 'Dr' was associated with atopic disease.40 In our study, AD patients with egg allergy exhibited a significantly higher frequency of functional group 'a' compared with AD patients without egg allergy or normal controls (P=0.033). A trend toward an association between egg allergy development in AD and functional group 'a' was observed, but significance was lost after Bonferroni correction. Further studies are needed to elucidate the association between HLA-DR functional groups and egg allergy.
Although no association between HLA-DRB1 polymorphisms and egg allergy was found in this study, several points should be taken into account. First, the study population was small. Given the number of HLA-DR alleles, it is difficult to obtain significant results using a small number of patients and controls owing to statistical limitations. Second, the diagnosis of egg allergy was not confirmed by oral food challenge. Controversy exists regarding the accuracy of serum-specific IgE levels in diagnosing food allergies.41 Third, multiple epitopes from various egg allergens may be bound by a number of discrete HLA class II molecules during processing and presentation, and it is possible that each allergen is associated with a specific HLA class II allele. The most important allergenic egg proteins are ovomucoid, ovalbumin, ovotransferrin, and lysozyme.42 Although ovalbumin is the most abundant protein in egg white, ovomucoid has been shown to be the dominant egg allergen.43 We did not confirm the presence of specific IgE against each egg allergenic protein in our patients. Finally, children who had outgrown an egg allergy might have been included in the AD without egg allergy group at enrollment. The selection of persistent egg allergy may assist in identifying alleles significantly associated with egg allergy.
In conclusion, the HLA-DRBI polymorphism was not associated with egg allergy. However, HLA-DRB1*11:01 was associated with AD in Korean children.
Figures and Tables
Table 1
DR restrictive supertype patterns designated by the polymorphic residues at positions 70, 71, and 74 of DRβ chain
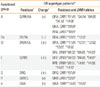
*Modified from Ou et al.27; †Polymorphic residues are presented as one-letter amino acid codes; ‡Overall charge of the DR restrictive supertype is indicated as positively charged (+), negatively charged (_), both positively and negatively charged (di-charged) (±), or uncharged (n).
References
1. Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998. 101:E8.
2. Guillet G, Guillet MH. Natural history of sensitizations in atopic dermatitis. A 3-year follow-up in 250 children: food allergy and high risk of respiratory symptoms. Arch Dermatol. 1992. 128:187–192.
3. Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population-based study in young children. Allergy. 2001. 56:403–411.
4. Dean T, Venter C, Pereira B, Arshad SH, Grundy J, Clayton CB, Higgins B. Patterns of sensitization to food and aeroallergens in the first 3 years of life. J Allergy Clin Immunol. 2007. 120:1166–1171.
5. Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long-term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006. 55:765–771.
6. Woo JG, Assa'ad A, Heizer AB, Bernstein JA, Hershey GK. The -159 C-->T polymorphism of CD14 is associated with nonatopic asthma and food allergy. J Allergy Clin Immunol. 2003. 112:438–444.
7. Campos E, Shimojo N, Inoue Y, Arima T, Suzuki S, Tomiita M, Matsuura T, Hata A, Suzuki Y, Aoyagi M, Kohno Y. No association of polymorphisms in the 5' region of the CD14 gene and food allergy in a Japanese population. Allergol Int. 2007. 56:23–27.
8. Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, Hermine O, Vijay S, Gambineri E, Cerf-Bensussan N, Fischer A, Ochs HD, Goulet O, Ruemmele FM. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007. 132:1705–1717.
9. Amoli MM, Hand S, Hajeer AH, Jones KP, Rolf S, Sting C, Davies BH, Ollier WE. Polymorphism in the STAT6 gene encodes risk for nut allergy. Genes Immun. 2002. 3:220–224.
10. Negoro T, Orihara K, Irahara T, Nishiyama H, Hagiwara K, Nishida R, Takagi H, Satoh K, Yamamoto Y, Shimizu S, Hagiwara T, Ishii M, Tanioka T, Nakano Y, Takeda K, Yoshimura I, Iikura Y, Tobe T. Influence of SNPs in cytokine-related genes on the severity of food allergy and atopic eczema in children. Pediatr Allergy Immunol. 2006. 17:583–590.
11. Kusunoki T, Okafuji I, Yoshioka T, Saito M, Nishikomori R, Heike T, Sugai M, Shimizu A, Nakahata T. SPINK5 polymorphism is associated with disease severity and food allergy in children with atopic dermatitis. J Allergy Clin Immunol. 2005. 115:636–638.
12. Campos Alberto EJ, Shimojo N, Suzuki Y, Mashimo Y, Arima T, Matsuura T, Inoue Y, Yamaide A, Tomiita M, Fujii K, Hata A, Kohno Y. IL-10 gene polymorphism, but not TGF-beta1 gene polymorphisms, is associated with food allergy in a Japanese population. Pediatr Allergy Immunol. 2008. 19:716–721.
13. Liu X, Beaty TH, Deindl P, Huang SK, Lau S, Sommerfeld C, Fallin MD, Kao WH, Wahn U, Nickel R. Associations between specific serum IgE response and 6 variants within the genes IL4, IL13, and IL4RA in German children: the German Multicenter Atopy Study. J Allergy Clin Immunol. 2004. 113:489–495.
14. Scherer MN, Graeb C, Tange S, Justl M, Jauch K, Geissler EK. Soluble allogeneic MHC class I molecule gene transfer promotes CTL apoptosis in vivo. Transplant Proc. 2001. 33:583–584.
15. Cardaba B, Cortegano I, Florido F, Arrieta I, Aceituno E, del Pozo V, Gallardo S, Rojo M, Palomino P, Lahoz C. Genetic restrictions in olive pollen allergy. J Allergy Clin Immunol. 2000. 105:292–298.
16. D'Amato M, Picardi A, Menna T, Di Somma C, Ariano R, di Pietro A, Charron D, Maggi E, Matricardi P, Plebani A, Poto S, Testa G, Sacerdoti G, Ruffilli A. HLA-DRB1* and allergy to Parietaria: linkage and association analyses. Hum Immunol. 1999. 60:1250–1258.
17. Sénéchal H, Geny S, Desvaux FX, Busson M, Mayer C, Aron Y, Oster JP, Bessot JC, Peltre G, Pauli G, Swierczewski E. Genetics and specific immune response in allergy to birch pollen and food: evidence of a strong, positive association between atopy and the HLA class II allele HLA-DR7. J Allergy Clin Immunol. 1999. 104:395–401.
18. Stephan V, Kuehr J, Seibt A, Saueressig H, Zingsem S, Dinh TD, Moseler M, Wahn V, Deichmann KA. Genetic linkage of HLA-class II locus to mite-specific IgE immune responsiveness. Clin Exp Allergy. 1999. 29:1049–1054.
19. Blumenthal MN. Positive association between HLA-DRB1*07 and specific IgE responses to purified major allergens of D. pteronyssinus (Der p 1 and Der p 2). Ann Allergy Asthma Immunol. 2002. 88:147–149.
20. Camponeschi B, Lucarelli S, Frediani T, Barbato M, Quintieri F. Association of HLA-DQ7 antigen with cow milk protein allergy in Italian children. Pediatr Allergy Immunol. 1997. 8:106–109.
21. Higgins JA, Lamb JR, Lake RA, O'Hehir RE. Polyclonal and clonal analysis of human CD4+ T-lymphocyte responses to nut extracts. Immunology. 1995. 84:91–97.
22. Hand S, Darke C, Thompson J, Stingl C, Rolf S, Jones KP, Davies BH. Human leucocyte antigen polymorphisms in nut-allergic patients in South Wales. Clin Exp Allergy. 2004. 34:720–724.
23. Rajka G. On definition and framework of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1989. 144:10–12.
24. Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996. 135:Suppl 48. 25–30.
25. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001. 107:891–896.
26. Bannai M, Tokunaga K, Lin L, Kuwata S, Mazda T, Amaki I, Fujisawa K, Juji T. Discrimination of human HLA-DRB1 alleles by PCR-SSCP (single-strand conformation polymorphism) method. Eur J Immunogenet. 1994. 21:1–9.
27. Ou D, Mitchell LA, Tingle AJ. A new categorization of HLA DR alleles on a functional basis. Hum Immunol. 1998. 59:665–676.
28. Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994. 368:215–221.
29. Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Fernández-Viña M, Geraghty DE, Holdsworth R, Hurley CK, Lau M, Lee KW, Mach B, Maiers M, Mayr WR, Müller CR, Parham P, Petersdorf EW, Sasazuki T, Strominger JL, Svejgaard A, Terasaki PI, Tiercy JM, Trowsdale J. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010. 75:291–455.
30. Ozawa A, Ohkido M, Tsuji K. Some recent advances in HLA and skin diseases. J Am Acad Dermatol. 1981. 4:205–230.
31. Schultz Larsen F, Grunnet N. Genetic investigations in atopic dermatitis. Tissue Antigens. 1987. 29:1–6.
32. Lee HJ, Ha SJ, Han H, Kim JW. Distribution of HLA-A, B alleles and polymorphisms of TAP and LMP genes in Korean patients with atopic dermatitis. Clin Exp Allergy. 2001. 31:1867–1874.
33. Saeki H, Kuwata S, Nakagawa H, Etoh T, Yanagisawa M, Miyamoto M, Tokunaga K, Juji T, Shibata Y. HLA and atopic dermatitis with high serum IgE levels. J Allergy Clin Immunol. 1994. 94:575–583.
34. Svejgaard E, Jakobsen B, Svejgaard A. Studies of HLA-ABC and DR antigens in pure atopic dermatitis and atopic dermatitis combined with allergic respiratory disease. Acta Derm Venereol Suppl (Stockh). 1985. 114:72–76.
35. Howell WM, Holgate ST. HLA genetics and allergic disease. Thorax. 1995. 50:815–818.
36. Boehncke WH, Loeliger C, Kuehnl P, Kalbacher H, Böhm BO, Gall H. Identification of HLA-DR and -DQ alleles conferring susceptibility to pollen allergy and pollen associated food allergy. Clin Exp Allergy. 1998. 28:434–441.
37. Shreffler WG, Charlop-Powers Z, Sicherer SH. Lack of association of HLA class II alleles with peanut allergy. Ann Allergy Asthma Immunol. 2006. 96:865–869.
38. Shinbara M, Kondo N, Agata H, Fukutomi O, Nishida T, Kobayashi Y, Orii T. T cell proliferation restricted by HLA class II molecules in patients with hen's egg allergy. Exp Clin Immunogenet. 1995. 12:103–110.
39. Fu XT, Bono CP, Woulfe SL, Swearingen C, Summers NL, Sinigaglia F, Sette A, Schwartz BD, Karr RW. Pocket 4 of the HLA-DR (alpha, beta 1*0401) molecule is a major determinant of T cells recognition of peptide. J Exp Med. 1995. 181:915–926.
40. Torres-Galván MJ, Quiralte J, Blanco C, Castillo R, Carrillo T, Pérez-Aciego P, Sánchez-García F. Pocket 4 in the HLA-DRB1 antigen-binding groove: an association with atopy. Allergy. 2000. 55:398–401.
41. van Nieuwaal NH, Lasfar W, Meijer Y, Kentie PA, Flinterman AE, Pasmans SG, Knulst AC, Hoekstra MO. Utility of peanut-specific IgE levels in predicting the outcome of double-blind, placebo-controlled food challenges. J Allergy Clin Immunol. 2010. 125:1391–1392.
42. Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994. 93:1047–1059.
43. Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997. 159:2026–2032.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download