Abstract
Purpose
Various therapeutic approaches have been suggested for preventing or reducing the adverse effects of topical glucocorticoids, including skin barrier impairment. Previously, we have shown that impairment of skin barrier function by the highest potency topical glucocorticoid, clobetasol 17-propinate (CP), can be partially prevented by co-application of a physiological lipid mixture containing pseudoceramide, free fatty acids, and cholesterol (multi-lamellar emulsion [MLE]). Skin atrophic effects of CP were also partially reduced by MLE. In this study, the preventive effects of MLE on the lowest potency topical glucocorticoid, hydrocortisone (HC), were investigated using animal models.
Methods
Anti-inflammatory activity of topical HC was evaluated using a 12-O-tetradecanoylphobol-13-acetate-induced skin edema model. Topical steroid induced adverse effects were evaluated using hairless mouse.
Results
The results showed that the anti-inflammatory activity was not altered by co-application of either MLE or hydrobase. However, co-application of MLE and 1.0% HC showed less impairment in the epidermal permeability barrier function, skin hydration, and skin surface pH compared with hydrobase. Stratum corneum integrity, evaluated by measuring trans-epidermal water loss after repeated tape stripping, showed less damage with MLE co-application. Long-term application of topical HC induced skin atrophy, measured by a reduction in skinfold and epidermal thickness and in the number of epidermal proliferating cell nucleus antigen (PCNA)-positive keratinocytes. Co-application of MLE did not affect the skinfold or epidermal thickness, but the number of PCNA-positive keratinocytes was less decreased with MLE use.
Currently, topical steroid treatment is considered a basic therapeutic regimen for various inflammatory skin diseases, ranging from acute contact dermatitis to chronic atopic dermatitis. However, even with their potent anti-inflammatory activity, a number of concerns exist around chronic and uncontrolled use of topical steroids due to local and systemic adverse effects.1 Concerns are especially high with respect to atopic dermatitis (AD) patients due to the chronic nature of the disease and relatively low age of patients. Topical steroids cause diverse local and systemic adverse effects, two of which are skin atrophy and systemic absorption. These adverse events are considered important reasons for the widespread steroid-phobia in AD patients.2,3 Previous research has also suggested that topical steroids result in a reduction in the synthesis of epidermal lipids, the precursors for the stratum corneum intercellular lipids, leading to impairment in skin barrier function.4,5 Disturbed skin barrier function is a major pathophysiological factor in AD,6 and further aggravation induced by topical steroids might result in a worsening of basal skin conditions.
In addition to the topical steroid, appropriate use of moisturizers is considered a baseline therapy for AD, as they can improve and reinforce the skin barrier function.7 Among the diverse components comprising the intercellular lipids, ceramide, cholesterol, and free fatty acids are the main constituents of the lamellar structure of intercellular lipids.8,9 This structure plays a major role in various barrier functions exerted by the skin, and it has been reported that a physiological lipid mixture (MLE), containing ceramide, cholesterol, and free fatty acids improves the skin barrier function.10 In addition, research groups, including ours, have reported that a pseudoceramide-containing MLE can improve skin barrier function.11-13
Various approaches have been suggested to overcome or minimize topical steroid-induced adverse effects including the application of physiological lipid mixtures.5 Topical steroids interfere with the epidermal synthesis of lipid precursors for the stratum corneum, and supplementation with a mixture of physiological lipids can alleviate the steroid-induced skin barrier function impairment. Previously, we have reported that MLE can reduce the adverse effects of the highest potency topical steroid, clobetasol 17-propionate (CP).14 However, relatively low-potency steroids are usually prescribed and used in AD.15 In this study, we investigated the effects of MLE on hydrocortisone (HC)-induced local adverse effects using animal models. HC (1%) was topically applied to normal skin of hairless mice, and either MLE or hydrobase was applied simultaneously. Skin barrier functions and skin atrophic effects were measured by non-invasive methods and histological observations. Anti-inflammatory activity of topical HC was also measured in a 12-O-tetradecanoylphobol-13-acetate-induced skin edema model using the ICR mouse.
Female hairless mice (hr-/hr-) (6 to 8 weeks old) and ICR mice (4 weeks old) (Hanlim Animal Center, Hwasung, Korea) were maintained under controlled humidity (50-60% relative humidity) and temperature (24℃±1℃) during the experimental period. The animals were fed a standard diet and tap water ad libitum. HC was dissolved in polyethylene glycol (molecular weight 400) and ethanol mixture (PEG400: EtOH=70:30) at 1% concentration (Chemicals purchased from Sigma-Aldrich Chemical, St. Louis, MO, USA). Preparation of pseudoceramide containing MLE was performed as previously reported.11 Stearic acid, cholesterol, and fatty acid triglycerides were obtained from Junsei Chemical (Tokyo, Japan). Glycerin monostearate, poly oxyethylene (15) glyceryl monostearate were obtained from Nihon Emulsion (Tokyo, Japan). Cetanol and squalane were purchased from Kao Co. (Tokyo, Japan) and Kishimoto (Hyogo, Japan), respectively. Pseudoceramide (myristyl/palmityl oxostearamide/arachiamide MEA: PC-9S) was acquired from NeoPharm Co., Ltd. (Daejeon, Korea). Hydrobase was prepared with spermaceti wax, stearyl alcohol, polyethanol glycol (Mw 4,000), glycerine, cetanol, and sodium lauryl sulphate.
Animal experiments were performed according to International Guiding Principles for Biomedical Research Involving Animals, and protocols were approved by the institutional review board of NeoPharm Co., Ltd. Anti-inflammatory activity of steroids was observed by topically applying 1.5 µg of 12-O-tetradecanoylphobol-13-acetate (TPA) (Sigma-Aldrich Chemical) in 20 µl of acetone (Sigma-Aldrich Chemical) to the right ear of the ICR mice. HC solution (30 mg of 1%) was topically applied to the same ear after 10 minutes, followed immediately by 30 mg of either MLE or hydrobase (n=7 for each group). Six hours after TPA application, the animals were sacrificed by cervical dislocation, and skin from the ear was taken using a 6-mm punch. The skin section was weighed, and anti-inflammatory activity was calculated as % of weight increase by topical treatment, compared with the control.
Steroid-induced local adverse effects were observed in a further experiment spanning a 6-day period of topical HC application on hairless mouse skin. Topical HC was applied once a day for 6 days, and either MLE or hydrobase was topically applied on the same site twice a day, at 10 minutes and 12 hours after the HC application. To evaluate the recovery of skin functions, the application of MLE or hydrobase was continued for 3 days after the completion of the topical steroid treatment. Epidermal permeability barrier function, skin hydration, and skin surface pH were evaluated by measuring trans-epidermal water loss (TEWL), skin surface capacitance, and skin surface pH, respectively. To achieve these measurements, a TEWameter TM300, a Corneometer CM 825, and a Skin-pH-Meter PH 905 were used (Equipment purchased from Courage & Khaza, Cologne, Germany). Skin atrophic effects were evaluated by measuring skinfold thickness. Three sites were randomly selected from each treated area, and skinfold thickness was measured using digital calipers (Mitutoyo, Tokyo, Japan).
To assess skin barrier integrity after steroid application, TEWL was measured after repeated tape stripping (10 times) with D-Squame (CuDerm, Dallas, TX, USA).
Skin biopsies were taken from the treated site post-HC treatment, fixed with 4% paraformaldehyde solution, and embedded in paraffin. Sections of 5 µm were prepared on probe-on-plus slides (Fisher Scientific, Pittsburg, PA, USA) and deparaffinized. Slides were stained using hematoxylin and eosin stain and immunohistochemical stain against proliferating cell nuclear antigen (PCNA) and viewed under light microscopy (Olympus BX51, Olympus Co., Tokyo, Japan).
To examine whether simultaneous application of pseudoceramide containing MLE with topical HC formulation affected the anti-inflammatory activity of the steroid, a TPA-induced skin edema model was used. Increased ear weight induced by topical application of TPA was significantly inhibited by topical HC. The inhibitory effects were equal for both the hydrobase and MLE co-applied sites, compared with HC only (Fig. 1). This result indicates that simultaneous application of MLE does not affect the anti-inflammatory activity of topical steroids.
The local adverse effects of topical glucocorticoids, including impairments on skin barrier functions, especially on epidermal permeability barrier function, skin hydration, and skin surface pH, were measured after simultaneous application with MLE. Epidermal permeability barrier function, assessed by measuring TEWL, increased after 6 days of topical HC application. This was partially prevented by co-application of MLE. The difference in TEWL between the two groups was statistically significant (P=0.016) (Fig. 2A). Reduction of skin hydration was also observed after 2 days of HC application, and the difference between the two groups was most evident at 3 days, but was not statistically significance. Recovery of skin hydration after discontinuation of the steroid was observed in both treatment groups (Fig. 2B). Skin surface pH gradually increased during steroid application, and at 6 days, the difference between the two groups was statistically significant (P=0.05). The skin surface pH was rapidly restored by 3 days post-steroid treatment (Fig. 2C). Skin barrier integrity was also evaluated by measuring TEWL after barrier disruption. Post-steroid application, tape stripping was performed on the application site and TEWL was measured. After 10 tape strips, the TEWL value was significantly higher in the hydrobase group compared with MLE group, suggesting that MLE improved the skin barrier integrity of steroid applied skin (Fig. 3).
The effect of topical HC on skin atrophy was investigated by measuring skinfold thickness during the application and histological analysis after steroid treatment. Significant reduction in skinfold thickness was observed after 3 days; however, the difference between the two groups was not significant for the test period. Although skin hydration and skin surface pH were restored after completion of the topical steroid treatment, recovery of skinfold thickness was not observed in the same time frame (Fig. 4A). Histological observations of the epidermal thickness showed slight but significant differences between the two groups (Fig. 4B, C, D). Immunohistochemical staining for PCNA confirmed the preventive effects of MLE on the inhibition of epidermal proliferation by topical steroids (Fig. 5).
We have shown that the co-application of pseudoceramide-containing physiological lipid mixture (multi-lamellar emulsion: MLE) with a low-potency topical corticosteroid reduces adverse effects, including impairments in skin barrier functions. Adverse events of topical steroids are usually local due to the low penetration of steroids through the skin.1 The local adverse effects include atrophic changes, increased infections, and miscellaneous effects such as acne, rosacea, hyper- or hypo-pigmentation, and rebound flares.1 To prevent or reduce these adverse effects, several approaches have been reported to be effective including the use of lower potency steroids or equipotent steroids with different potency of skin atrophy,16 alteration of application scheme, such as alternate-day therapy,17 or co-application with topical tretinoin.18 Impairment of skin barrier functions by topical steroids, expressed as increased TEWL, decreased skin hydration, and structural disorganization of the stratum corneum intercellular lipids have been reported in vivo and through clinical studies.4,5 Several reports have suggested that skin barrier impairment by topical steroids is due to structural disorganization of the stratum corneum intercellular lipid lamellar structure.19 In addition, the increase in skin surface pH observed after topical steroid treatment is due to the decreased synthesis of free fatty acid precursors in viable epidermis.14
The stratum corneum, the outermost layer of the skin, plays the most important role in barrier functions, including epidermal permeability, hydration, and anti-microbial functions.9 The most widely used structural model for the stratum corneum is the 'brick and mortar' model, describing the corneocytes as 'bricks' and intercellular lipids as 'mortar.'8 Previous studies have suggested that the structural arrangement of intercellular lipids into the distinctive lamellar structure is the most important factor for those barrier functions, and defects or disorganization of the lamellar structure results in impaired skin barrier functions.20 Several precursor molecules comprising the intercellular lipids are synthesized in the keratinocytes in viable epidermis and transported into the stratum corneum layer through the lamellar bodies. Extracellular processing of the precursor molecules in the stratum corneum produces the lipid components for the lamellar structure. Due to the inhibitory effects of topical steroids on the lipid precursor synthesis in keratinocytes, impairments of the lamellar structure in the stratum corneum are induced, resulting in a disturbance of skin barrier function. In previous reports, an equimolar mixture of the major constituents of human stratum corneum intercellular lipids, i.e., free fatty acid, cholesterol, and ceramide, showed beneficial effects in restoring the permeability barrier functions and stratum corneum integrity.5 Although the highest potency topical steroid, CP was used in previous studies and the lowest potency topical steroid, HC, was used in this study, similar results were observed for skin barrier functions. Increase of TEWL, decrease of skin hydration, increase of skin surface pH, and disturbed stratum corneum integrity were observed after 6 days of topical HC application. Consistent with previous studies, co-application of pseudoceramide containing MLE resulted in nearly identical lamellar structure in the naive human stratum corneum, preventing topical steroid-induced adverse effects.
AD, characterized by impaired skin barrier function and immunologic disturbance, is a chronic inflammatory skin disease and usually requires a long treatment duration.21 Diverse therapeutic regimens have been used for the treatment of AD, but the cornerstone therapy is moisturizers and topical steroids. Physiological lipid mixtures, due to their efficacy of improving skin barrier function, have been suggested to have beneficial effects on the management of AD.22 Because the skin barrier function of AD patients is already compromised, topical steroid treatment can interfere or cause the deterioration of the skin barrier function, which necessitates the use of skin barrier-enhancing moisturizers. Along with improvements in the skin barrier function, these results confirm the beneficial effects of physiological lipid mixtures in reducing topical steroid-induced adverse effects.
Consistent with a previous study, as well as skin barrier function improvement, skin atrophy was slightly prevented by MLE. Demerjian et al.23 reported that the activator of peroxisome proliferator-activated receptor (PPAR)-α, PPARβ/δ and liver X receptor (LXR) can partially prevent the decrease in keratinocyte proliferation in topical steroid treated murine skin. PPARα, β/δ, and LXR activators are known to exert diverse effects on epidermal structure and functions, including anti-inflammatory effects. Moreover, the activators also improve the epidermal permeability barrier function, mainly due to the stimulating activity on the epidermal lipid synthesis, which is required for stratum corneum intercellular lipid formation. In addition, PPAR and LXR activators also regulate keratinocyte proliferation.24 In this study, myristyl/palmitoyl oxostearamide/arachinamide MEA (PC-9S) was used as a pseudoceramide for physiological lipid preparation. The chemical structure of PC-9S is similar to that of palmitoylethanolamine, which was previously reported as having PPAR activating effects,25 and according to our preliminary studies, PC-9S showed significant PPAR activating effect in cultured human keratinocytes. Although further investigation is required, the PPAR-activating effects of PC-9S might be a possible explanation for the beneficial effects of MLE.
In conclusion, our study showed that the co-application of MLE prevents topical steroid-induced adverse effects. Skin barrier function impairments and inhibition of keratinocyte proliferation were partially reduced by MLE. These results suggest that the use of physiological lipid mixtures with topical steroids can be a beneficial therapeutic option, especially for AD patients.
Figures and Tables
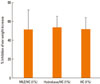 | Fig. 1Anti-inflammatory activity of topical hydrocortisone (HC) with multi-lamellar emulsion (MLE) and hydrobase. Co-application of either MLE or hydrobase did not inhibit ear weight increase by TPA application, compared with HC only treatment. MLE or hydrobase was applied 10 min after TPA application. After 6 hr, ear skin was taken with a 6-mm punch, tissue weight was measured, and inhibitory activity was calculated. |
 | Fig. 2Change of skin functions during topical steroid treatment. Topical hydrocortisone treatment-induced skin barrier impairments, represented by an increase in trans-epidermal water loss (A), reduction of skin hydration (B), and increase in skin surface pH (C). After discontinuation of steroid treatment, restoration of skin hydration and skin surface pH were observed after 3 days. Co-application of pseudoceramide-containing multi-lamellar emulsion showed less impairment in all the measured parameters compared with hydrobase. |
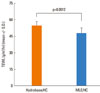 | Fig. 3Skin barrier integrity assessed by change of trans-epidermal water loss (TEWL) after tape stripping. After 10 strips, TEWL was significantly higher in the hydrobase co-applied site compared with the multi-lamellar emulsion (MLE) co-applied site. |
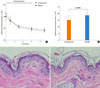 | Fig. 4Skin atrophic effects of topical hydrocortisone. During topical treatment, skinfold thickness was measured (A), and after 6 days of steroid treatment, skin biopsy was taken and histological analysis performed. Reduction of epidermal thickness was observed in both groups (normal epidermal thickness represented by dotted line) (B) the multi-lamellar emulsion co-applied site (C) showed less reduction in epidermal thickness compared with hydrobase (D). |
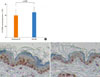 | Fig. 5Reduction of epidermal proliferation by topical hydrocortisone (HC) treatment. proliferating cell nucleus antigen (PCNA) staining on skin biopsy samples showed a decrease in the number of PCNA-positive keratinocytes in the basal layer after 6 days of HC treatment compared with that in normal skin (represented by dotted line) (A). Multi-lamellar emulsion (MLE) co-application partially prevented the reduction of PCNA-positive keratinocytes (B) compared with hydrobase (C). |
References
1. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006. 54:1–15.
2. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000. 142:931–936.
3. Fukaya M. Why do patients with atopic dermatitis refuse to apply topical corticosteroids? Dermatology. 2000. 201:242–245.
4. Sheu HM, Lee JY, Chai CY, Kuo KW. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. Br J Dermatol. 1997. 136:884–890.
5. Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, Ahn SK, Brown BE, Elias PM, Feingold KR. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003. 120:456–464.
6. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008. 121:1337–1343.
7. Proksch E. The role of emollients in the management of diseases with chronic dry skin. Skin Pharmacol Physiol. 2008. 21:75–80.
8. Lee SH, Jeong SK, Ahn SK. An update of the defensive barrier function of skin. Yonsei Med J. 2006. 47:293–306.
9. Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008. 8:299–305.
10. Mao-Qiang M, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol. 1996. 106:1096–1101.
11. Park BD, Youm JK, Jeong SK, Choi EH, Ahn SK, Lee SH. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type III synthetic ceramide. J Invest Dermatol. 2003. 121:794–801.
12. Rim JH, Jo SJ, Park JY, Park BD, Youn JI. Electrical measurement of moisturizing effect on skin hydration and barrier function in psoriasis patients. Clin Exp Dermatol. 2005. 30:409–413.
13. Draelos ZD. The effect of ceramide-containing skin care products on eczema resolution duration. Cutis. 2008. 81:87–91.
14. Ahn SK, Bak HN, Park BD, Kim YH, Youm JK, Choi EH, Hong SP, Lee SH. Effects of a multilamellar emulsion on glucocorticoid-induced epidermal atrophy and barrier impairment. J Dermatol. 2006. 33:80–90.
15. Beattie PE, Lewis-Jones MS. Parental knowledge of topical therapies in the treatment of childhood atopic dermatitis. Clin Exp Dermatol. 2003. 28:549–553.
16. Korting HC, Unholzer A, Schafer-Korting M, Tausch I, Gassmueller J, Nietsch KH. Different skin thinning potential of equipotent medium-strength glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2002. 15:85–91.
17. Tharp MD. A comparison of twice-daily and once-daily administration of fluticasone propionate cream, 0.05%, in the treatment of eczema. Cutis. 1996. 57:19–26.
18. Kaidbey K, Kopper SC, Sefton J, Gibson JR. A pilot study to determine the effect of tazarotene gel 0.1% on steroid-induced epidermal atrophy. Int J Dermatol. 2001. 40:468–471.
19. Sheu HM, Lee JY, Kuo KW, Tsai JC. Permeability barrier abnormality of hairless mouse epidermis after topical corticosteroid: characterization of stratum corneum lipids by ruthenium tetroxide staining and high-performance thin-layer chromatography. J Dermatol. 1998. 25:281–289.
20. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.
21. Abramovits W. Atopic dermatitis. J Am Acad Dermatol. 2005. 53:S86–S93.
22. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009. 9:437–446.
23. Demerjian M, Choi EH, Man MQ, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol. 2009. 18:643–649.
24. Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008. 49:499–509.
25. Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005. 67:15–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download